GR-89696
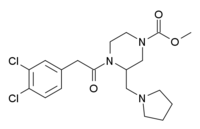 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
126766-32-3 |
| PubChem (CID) | 3505 |
| IUPHAR/BPS | 1649 |
| ChEMBL |
CHEMBL277863 |
| Chemical and physical data | |
| Formula | C19H25Cl2N3O3 |
| Molar mass | 414.325 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| | |
GR-89696 is a drug which acts as a highly selective κ-opioid agonist.[1] It shows selective effects in different animal models and it is thought it may be a subtype-selective agonist for the κ2 subtype.[2][3][4] Recent studies have suggested that GR-89696 and related κ2-selective agonists may be useful for preventing the itching which is a common side effect of conventional opioid analgesic drugs, without the additional side effects of non-selective kappa agonists.[5]
References
- ↑ Naylor A, Judd DB, Lloyd JE, Scopes DI, Hayes AG, Birch PJ (July 1993). "A potent new class of kappa-receptor agonist: 4-substituted 1-(arylacetyl)-2-[(dialkylamino)methyl]piperazines". Journal of Medicinal Chemistry. 36 (15): 2075–83. doi:10.1021/jm00067a004. PMID 8393489.
- ↑ Herrero JF, Headley PM (September 1993). "Functional evidence for multiple receptor activation by kappa-ligands in the inhibition of spinal nociceptive reflexes in the rat". British Journal of Pharmacology. 110 (1): 303–9. doi:10.1111/j.1476-5381.1993.tb13809.x. PMC 2176008
 . PMID 8220893.
. PMID 8220893. - ↑ Ho J, Mannes AJ, Dubner R, Caudle RM (April 1997). "Putative kappa-2 opioid agonists are antihyperalgesic in a rat model of inflammation". The Journal of Pharmacology and Experimental Therapeutics. 281 (1): 136–41. PMID 9103490.
- ↑ Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH (September 2001). "GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 298 (3): 1049–59. PMID 11504802.
- ↑ Ko MC, Husbands SM (January 2009). "Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates". The Journal of Pharmacology and Experimental Therapeutics. 328 (1): 193–200. doi:10.1124/jpet.108.143925. PMC 2719014
 . PMID 18842704.
. PMID 18842704.
This article is issued from Wikipedia - version of the 5/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.