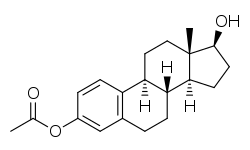
Estradiol acetate
 | |
| Clinical data | |
|---|---|
| Pronunciation | ESS-tra-DYE-ole ass-uh-tate[1] |
| Trade names | Femtrace, Femring, Menoring |
| Routes of administration | Oral, vaginal (ring) |
| ATC code | G03CA03 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 4245-41-4 |
| PubChem (CID) | 9818306 |
| DrugBank | DBSALT000065 |
| ChemSpider | 7994056 |
| UNII | 5R97F5H93P |
| KEGG | D04061 |
| ChEMBL | CHEMBL1200430 |
| Chemical and physical data | |
| Formula | C20H26O3 |
| Molar mass | 314.419 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Estradiol acetate (INN, USAN, BAN) (brand names Femtrace, Femring, Menoring), or estradiol 3-acetate, is a semisynthetic, steroidal estrogen and an estrogen ester – specifically, the 3-acetate ester of estradiol.[2] It acts as a prodrug of estradiol, and hence, is considered to be a natural, bioidentical form of estrogen.[3] Estradiol acetate is relatively recent to the market, having been first approved in a vaginal ring formulation as Menoring in the United Kingdom in 2001,[4] followed by another vaginal ring formulation as Femring in the United States in 2003,[5] and finally as an oral preparation as Femtrace in the United States in 2004.[5] It is used as a component of hormone replacement therapy to treat and prevent symptoms of menopause such as osteoporosis in postmenopausal women.[4][6][7][8]
The Women's Health Initiative studies report increased health risks for postmenopausal women when using unopposed estrogens. Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.[9]
See also
References
- ↑ https://www.drugs.com/cdi/estradiol-acetate.html
- ↑ Buckler H, Al-Azzawi F (2003). "The effect of a novel vaginal ring delivering oestradiol acetate on climacteric symptoms in postmenopausal women". BJOG. 110 (8): 753–9. PMID 12892687.
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 261–. ISBN 978-3-642-60107-1.
- 1 2 Speroff L (October 2003). "Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms". Obstetrics and Gynecology. 102 (4): 823–34. doi:10.1016/s0029-7844(03)00764-6. PMID 14551014.
- 1 2 Sivanandy MS, Masimasi N, Thacker HL (May 2007). "Newer hormonal therapies: lower doses; oral, transdermal, and vaginal formulations". Cleveland Clinic Journal of Medicine. 74 (5): 369–75. doi:10.3949/ccjm.74.5.369. PMID 17506242.
- ↑ Al-Azzawi F, Lees B, Thompson J, Stevenson JC (2005). "Bone mineral density in postmenopausal women treated with a vaginal ring delivering systemic doses of estradiol acetate". Menopause (New York, N.Y.). 12 (3): 331–9. doi:10.1097/01.gme.0000163870.03388.4d. PMID 15879923.
- ↑ Utian WH, Speroff L, Ellman H, Dart C (2005). "Comparative controlled trial of a novel oral estrogen therapy, estradiol acetate, for relief of menopause symptoms". Menopause (New York, N.Y.). 12 (6): 708–15. doi:10.1097/01.gme.0000184220.63459.a8. PMID 16278614.
- ↑ Speroff L, Haney AF, Gilbert RD, Ellman H (2006). "Efficacy of a new, oral estradiol acetate formulation for relief of menopause symptoms". Menopause (New York, N.Y.). 13 (3): 442–50. doi:10.1097/01.gme.0000182802.06762.b2. PMID 16735941.
- ↑ "FEMRING". DailyMed. U.S. National Library of Medicine. Retrieved June 2010. Check date values in:
|access-date=(help)