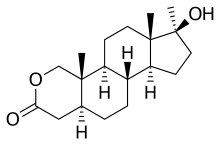
Oxandrolone
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604024 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | A14AA08 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Metabolism | Hepatic |
| Biological half-life | 9 hours |
| Excretion | Urinary:90%; Fecal:7% |
| Identifiers | |
| |
| CAS Number |
53-39-4 |
| PubChem (CID) | 5878 |
| IUPHAR/BPS | 7092 |
| DrugBank |
DB00621 |
| ChemSpider |
5667 |
| UNII |
7H6TM3CT4L |
| KEGG |
D00462 |
| ChEBI |
CHEBI:7820 |
| ChEMBL |
CHEMBL1200436 |
| Chemical and physical data | |
| Formula | C19H30O3 |
| Molar mass | 306.44 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Oxandrolone (brand names Oxandrin, Anavar, Lonavar, others), is a synthetic, orally active anabolic-androgenic steroid (AAS) that first become available as a prescription drug in the United States in 1964. It is a 17α-methylated derivative of dihydrotestosterone (DHT) that has an oxygen atom in place of the carbon at the 2 position.
Researchers and medical professionals have used oxandrolone to treat a wide variety of disorders. These include idiopathic short stature, Turner syndrome, body mass loss from catabolic illness or long-term corticosteroid treatment, severe burns, surgical or general trauma, osteoporosis, anemia, hereditary angioedema, HIV/AIDS-induced wasting, alcoholic hepatitis, and hypogonadism.[1]
Oxandrolone is well-established as a safe treatment for patients recovering from severe burns.[2][3] Medical research has also established oxandrolone's efficacy in aiding the development of girls with Turner syndrome. Although oxandrolone has long been used to accelerate growth in children with idiopathic short stature, it is unlikely to increase adult height and in some cases may even decrease it. Oxandrolone has therefore largely been replaced by growth hormone for this use.[4]
Some bodybuilders use oxandrolone for its muscle-building properties, usually purchasing it from black market suppliers. This is illegal in the United States, Canada, the United Kingdom, and many other countries.
Medical uses
Oxandrolone has been researched and prescribed as a treatment for a wide variety of conditions. Oxandrolone is FDA-approved for treating osteoporosis, aiding weight gain, and counteracting the catabolic effect of long-term corticosteroid treatment.[5][6] As of 2016, it is often prescribed off-label to quicken recovery from severe burns, aid the development of girls with Turner syndrome, and counteract HIV/AIDS-induced wasting.
Oxandrolone increases both short-term and long-term outcomes in patients recovering from severe burns. Most evidence shows that it reduces the amount of time spent hospitalized and that improved muscle and bone recovery are still measurable a year after injury.[2][3]
Research suggests that oxandrolone is also effective in the treatment of alcoholic hepatitis.[7]
Contraindications
Like other anabolic steroids, oxandrolone may worsen hypercalcemia by increasing osteolytic bone resorption.[5] When taken by pregnant women, oxandrolone may have unintended effects such as masculinization on the fetus.[5]
Side effects
Although some orally-administered anabolic steroids are hepatotoxic, oxandrolone causes little or no detectable stress to burn victims' livers at clinical doses.[2] This supports the notion common among bodybuilders that oxandrolone is less hepatotoxic than most common oral anabolic steroids.[8]
Women who are administered oxandrolone may experience virilization, the non-reversible development of masculine features such as voice deepening, hirsutism, menstruation abnormalities, male-pattern hair loss, and clitoral enlargement.[4][9][5] Oxandrolone may disrupt growth in children, reducing their adult height.[10] Because of these side effects, doses given to women and children are chosen carefully and patients are usually monitored for virilization and growth abnormalities.[4][9]
Unlike some anabolic steroids, oxandrolone does not generally cause gynecomastia because it is not aromatized into estrogenic metabolites.[11]
Like other androgens, oxandrolone can cause or worsen acne and priapism (unwanted or prolonged erections).[10][5] Oxandrolone can also reduce males' fertility, another side effect common among androgens.[10] In an attempt to compensate for the exogenous increase in androgens, the body may reduce testosterone production via testicular atrophy and inhibition of gonadotropic activity.[5]
Interactions
Oxandrolone greatly increases warfarin's blood-thinning effect, sometimes dangerously so.[12] In April 2004, Savient Pharmaceuticals published a safety alert through the FDA warning healthcare professionals of this.[13] Oxandrolone can also inhibit the metabolism of oral hypoglycemic agents.[5] It may worsen edema when taken alongside adrenal cortical steroids or adrenocorticotropic hormone (ACTH).[5]
Pharmacology
Mechanism of action
| Preparation | Ratio |
|---|---|
| Testosterone | 1:1 |
| Testosterone cypionate | 1:1 |
| Testosterone enanthate | 1:1 |
| Methyltestosterone | 1:1 |
| Fluoxymesterone | 1:2 |
| Oxymetholone | 1:3 |
| Oxandrolone | 1:3–1:13 |
| Nandrolone decanoate | 1:2.5–1:4 |
Like other anabolic steroids, oxandrolone is an androgen receptor agonist. This increases protein synthesis, which increases muscle growth, lean body mass, and bone mineral density.[3]
Compared to testosterone and most other anabolic steroids, oxandrolone is less androgenic relative to its strength as an anabolic.[14] This often motivates its medical use in children and women because less androgenic effect implies less risk of virilization. The bodybuilding community also considers this fact when choosing between steroids.[8]
Dosage
Children with idiopathic short stature or Turner syndrome are given doses of oxandrolone far smaller than those given to burn patients. Researchers have chosen dose ranges for children with great care in order to minimize the likelihood of virilization and premature maturation.[4][9] Most adults treated with oxandrolone receive moderate doses.[2][7][11] Bodybuilders often take doses much higher than these.
Oxandrolone is generally administered at least once daily.
History
Oxandrolone was first made by Raphael Pappo and Christopher J. Jung while at Searle Laboratories (now part of Pfizer). The researchers first described the chemical in 1962.[15][16] They were immediately interested in oxandrolone's very weak androgenic effect relative to its anabolic effect.[15] It was released as a pharmaceutical drug in the United States in 1964.
The drug was prescribed to promote muscle regrowth in disorders which cause involuntary weight loss, and is used as part of treatment for HIV/AIDS. It had also been shown to be partially successful in treating cases of osteoporosis. However, in part due to bad publicity from its illicit use by bodybuilders, production of Anavar was discontinued by Searle Laboratories in 1989. It was picked up by Bio-Technology General Corporation, which changed its name to Savient Pharmaceuticals who, following successful clinical trials in 1995, released it under the tradename Oxandrin.[8] BTG subsequently won approvals for orphan drug status by the Food and Drug Administration (FDA) for treating alcoholic hepatitis, Turner syndrome, and HIV-induced weight loss. It is also indicated as an offset to protein catabolism caused by long-term administration of corticosteroids.
Society and culture
Legal status
In the United States, oxandrolone is categorized as a Schedule III controlled substance under the Controlled Substances Act along with many other anabolic steroids.[17] It is a Schedule IV controlled substance in Canada and a Schedule 4 Controlled Drug in the United Kingdom.
Non-medical use
Many bodybuilders and athletes use oxandrolone for its muscle-building properties. Oxandrolone is much less androgenic than anabolic, so women and those seeking less intense steroid regimens use it particularly often.[8] Many also value oxandrolone's low hepatotoxicity relative to most other orally active steroids.[8]
Brand names
The original brand name of oxandrolone was Anavar. Oxandrin is often used as a generic trade name. Oxandrolone has also been sold under the trade names Lonavar (Argentina, Australia), Lipidex (Brazil), Antitriol (Spain), Anatrophill (France), Protivar, and Vasorome.[8][18] Additional brand names exist for products that are manufactured for the steroid black market.[8]
References
- ↑ Bork, Konrad (2012). "Current Management Options for Hereditary Angioedema". Current Allergy and Asthma Reports. 12 (4): 273–280. doi:10.1007/s11882-012-0273-4. ISSN 1529-7322.
- 1 2 3 4 Li, Hui; Guo, Yinan; Yang, Zhenyu; Roy, Mridul; Guo, Qulian (2016). "The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis". Burns. 42 (4): 717–727. doi:10.1016/j.burns.2015.08.023. ISSN 0305-4179.
- 1 2 3 Rojas, Yesenia; Finnerty, Celeste C; Radhakrishnan, Ravi S; Herndon, David N (2012). "Burns: an update on current pharmacotherapy". Expert Opinion on Pharmacotherapy. 13 (17): 2485–2494. doi:10.1517/14656566.2012.738195. ISSN 1465-6566.
- 1 2 3 4 Wit, Jan M.; Oostdijk, Wilma (2015). "Novel approaches to short stature therapy". Best Practice & Research Clinical Endocrinology & Metabolism. 29 (3): 353–366. doi:10.1016/j.beem.2015.01.003. ISSN 1521-690X.
- 1 2 3 4 5 6 7 8 "Oxandrolone Tablets, USP - Rx only" (PDF). Drugs@FDA. U.S. Food and Drug Administration. 1 December 2006. Retrieved 21 June 2016.
- ↑ "Oxandrin (oxandrolone tablets, USP)" (PDF). Drugs@FDA. BTG Pharmaceuticals, U.S. Food and Drug Administration. 21 April 2003. Retrieved 21 June 2016.
- 1 2 Choi, Gina; Runyon, Bruce Allen (2012). "Alcoholic Hepatitis: A Clinician's Guide". Clinics in Liver Disease. 16 (2): 371–385. doi:10.1016/j.cld.2012.03.015. ISSN 1089-3261.
- 1 2 3 4 5 6 7 Llewellyn, William (2011). William Llewellyn's Anabolics. Jupiter, FL: Molecular Nutrition, LLC. pp. 610–616. ISBN 978-0-9828280-1-4.
- 1 2 3 Sas, T.C.J.; Gault, E.J.; Zeger Bardsley, M.; Menke, L.A.; Freriks, K.; Perry, R.J.; Otten, B.J.; de Muinck Keizer-Schrama, S.M.P.F.; Timmers, H.; Wit, J.M.; Ross, J.L.; Donaldson, M.D.C. (2014). "Safety and Efficacy of Oxandrolone in Growth Hormone-Treated Girls with Turner Syndrome: Evidence from Recent Studies and Recommendations for Use". Hormone Research in Paediatrics. 81 (5): 289–297. doi:10.1159/000358195. ISSN 1663-2826.
- 1 2 3 "Oxandrolone". MedlinePlus. The American Society of Health-System Pharmacists, Inc. 15 May 2016. Retrieved 21 June 2016.
- 1 2 Corona, Giovanni; Rastrelli, Giulia; Vignozzi, Linda; Maggi, Mario (2012). "Emerging medication for the treatment of male hypogonadism". Expert Opinion on Emerging Drugs. 17 (2): 239–259. doi:10.1517/14728214.2012.683411. ISSN 1472-8214.
- ↑ Demling, Robert H. (September 2004). "Oxandrolone (Oxandrin) use and the interaction with warfarin" (PDF). U.S. Food and Drug Administration. Retrieved 20 June 2016.
- ↑ Ottery, Faith D. (20 April 2004). "Oxandrin (oxandrolone) Dear Healthcare Professional Letter Apr 2004". Safety Alerts for Human Medical Products. U.S. Food and Drug Administration. Retrieved 20 June 2016.
- 1 2 Chrousos, George P. (2012). "The Gonadal Hormones & Inhibitors". In Katzung, Bertram G. Basic & Clinical Pharmacology. New York London: McGraw-Hill Medical McGraw-Hill distributor. p. 735. ISBN 0071764011.
- 1 2 Pappo, Raphael; Jung, Christopher J. (1962). "2-oxasteroids: A new class of biologically active compounds". Tetrahedron Letters. 3 (9): 365–371. doi:10.1016/S0040-4039(00)70883-5. ISSN 0040-4039.
- ↑ Fox, Maurice; Minot, Ann S.; Liddle, Grant W. (1962). "Oxandrolone: A Potent Anabolic Steroid of Novel Chemical Configuration". The Journal of Clinical Endocrinology & Metabolism. 22 (9): 921–924. doi:10.1210/jcem-22-9-921. ISSN 0021-972X.
- ↑ "Controlled Substances Act". United States Food and Drug Administration. 11 June 2009. Retrieved 17 June 2016.
- ↑ Drugs of Abuse (PDF). United States Drug Enforcement Administration. 2011. p. 22.
External links
- Oxandrin Homepage, savientpharma.com (via archive.org)
- Oxandrin Label, fda.gov (retrieved 23 October 2009)
- "Oxandrolone Side Effects, Interactions and Information". drugs.com.