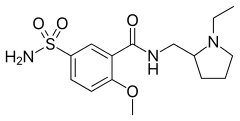
Sulpiride
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eglonyl, Dolmatil, Sulpor |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets, capsules, oral solution), IM |
| ATC code | N05AL01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25–40%[1][2] |
| Protein binding | <40%[1] |
| Metabolism | Not metabolised[3] |
| Biological half-life | 6-8 hours[1] |
| Excretion | Renal (70–90%),[2] Fecal (~95% as the unchanged drug)[1] |
| Identifiers | |
| |
| CAS Number |
15676-16-1 |
| PubChem (CID) | 5355 |
| IUPHAR/BPS | 5501 |
| DrugBank |
DB00391 |
| ChemSpider |
5162 |
| UNII |
7MNE9M8287 |
| KEGG |
D01226 |
| ChEMBL |
CHEMBL26 |
| ECHA InfoCard | 100.036.124 |
| Chemical and physical data | |
| Formula | C15H23N3O4S |
| Molar mass | 341.427 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Sulpiride (brand names Dogmatil (HK, SG, PH), Dolmatil (IE, UK), Eglonyl (RU, ZA), Espiride (ZA), Modal (IL), Prometar (UY), Sulpor (UK) and others) is an atypical antipsychotic drug (although some texts have referred to it as a typical antipsychotic[4]) of the benzamide class used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and sometimes used in low dosage to treat anxiety and mild depression. Sulpiride is commonly used in Europe, Russia and Japan. Levosulpiride is its purified levo-isomer and is sold in India for similar purpose. So far it has not been approved in the United States, Canada and Australia. The drug is chemically and clinically similar to amisulpride.
Medical uses
Sulpiride's primary use in medicine is in the management of the symptoms of schizophrenia.[1] It has been used as both a monotherapy and adjunctive therapy (in case of treatment-resistance) in schizophrenia.[1][5][6][7][8][9] It has also been used in the treatment of dysthymia.[10] Augmentation with sulpiride has also been tried as a strategy for accelerating antidepressant response in patients with major depressive disorder.[11] There is also evidence of its efficacy in treating panic disorder.[12][13]
Pregnancy and lactation
- Pregnancy: Animal studies did not reveal any embryotoxicity or fetotoxicity, nor did limited human experience. Due to insufficient human data, pregnant women should be treated with sulpiride only if strictly indicated. Additionally, the newborns of treated women should be monitored, because isolated cases of extrapyramidal side effects have been reported.[1]
- Lactation: Sulpiride is found in the milk of lactating women. Since the consequences are unclear, women should not breastfeed during treatment.[1]
Adverse effects
Sulpiride is usually well-tolerated, producing few adverse effects. Their incidences are as follows:[1][5][14][15][16][17][18][19][20]
- Common (>1%) adverse effects
- Dizziness
- Headache
- Extrapyramidal side effects
- - Tremor
- - Dystonia
- - Akathisia — a sense of inner restlessness that presents itself with the inability to stay still
- - Parkinsonism
- Somnolence (not a very prominent adverse effect considering its lack of α1 adrenergic, histamine and muscarinic acetylcholine receptor affinity)
- Insomnia
- Weight gain or loss
- Hyperprolactinemia (elevated plasma levels of the hormone, prolactin which can, in turn lead to sexual dysfunction, galactorrhea, amenorrhea, gynecomastia, etc.)
- Nausea
- Vomiting
- Nasal congestion
- Anticholinergic adverse effects such as:
- - Dry mouth
- - Constipation
- - Blurred vision
- Impaired concentration
- Rare (<1% incidence) adverse effects
- Tardive dyskinesia — a rare, often permanent movement disorder that, more often than not, results from prolonged treatment with antidopaminergic agents such as antipsychotics. It presents with slow (hence tardive), involuntary, repetitive and purposeless movements that most often affect the facial muscles.
- Neuroleptic malignant syndrome — a rare, life-threatening complication that results from the use of antidopaminergic agents. Its incidence increases with concomitant use of lithium (medication) salts
- Blood dyscrasias — rare, sometimes life-threatening complications of the use of a number of different antipsychotics (most notably clozapine) which involves abnormalities in the composition of a person's blood (e.g. having too few white blood cells per unit volume of blood). Examples include:
- - Agranulocytosis — a significant drop in white blood cell count, leaving individuals wide open to life-threatening opportunistic infections
- - Neutropenia
- - Leucopenia
- - Leukocytosis[21]
- Seizures
- Torsades de pointes
- Unknown incidence adverse effects include
- QTc interval prolongation which can lead to potentially fatal arrhythmias.
- Cholestatic jaundice[22]
- Elevated liver enzymes
- Primary biliary cirrhosis[23]
- Allergic reactions
- Photosensitivity — sensitivity to light
- Skin rashes
- Depression
- Catatonia
- Palpitations
- Agitation
- Diaphoresis — sweating without a precipitating factor (e.g. increased ambient temperature)
- Hypotension — low blood pressure
- Hypertension — high blood pressure
- Venous thromboembolism (probably rare)
Contraindications and cautions
Contraindications[1]
- Hypersensitivity to sulpiride
- Pre-existing breast cancer or other prolactin-dependent tumors
- Phaeochromocytoma
- Intoxication with other centrally-active drugs
- Concomitant use of levodopa
- Acute porphyria
- Comatose state or CNS depression
- Bone-marrow suppression
Cautions[1]
- Pre-existing Parkinson's Disease
- Patients below 18 years of age (insufficient clinical data)
- Pre-existing severe heart disease/bradycardia, or hypokalemia (predisposing to long QT syndrome and severe arrhythmias)
- Patients with pre-existing epilepsy. Anticonvulsant therapy should be maintained
- Lithium use — increased risk of neurological side effects of both drugs
Overdose
Sulpiride has a relatively low order of acute toxicity. Substantial amounts may cause severe but reversible dystonic crises with torticollis, protrusion of the tongue, and/or trismus. In some cases all the classical symptoms typical of severe Parkinson's disease may be noted; in others, over-sedation/coma may occur. The treatment is largely symptomatic. Some or all extrapyramidal reactions may respond to the application of anticholinergic drugs such as biperiden or benzatropine. All patients should be closely monitored for signs of long QT syndrome and severe arrhythmias.
Synthesis
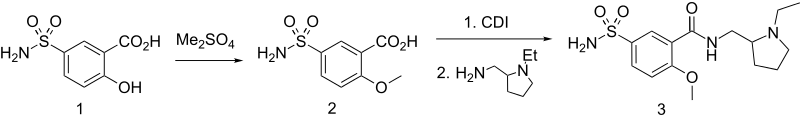
Sulpiride can be synthesized from 5-aminosulfosalicylic acid. Methylating this with dimethylsulfate gives 2-methoxy-5-aminosulfonylbenzoic acid, which is transformed into an amide using 2-aminomethyl-1-ethylpyrrolidine as the amine component and carbonyldiimidazole (CDI) as a condensing agent.
Pharmacology
Sulpiride is a selective antagonist at dopamine D2 and D3 receptors. This action dominates in doses exceeding 600 mg daily. In doses of 600 to 1,600 mg sulpiride shows mild sedating and antipsychotic activity. Its antipsychotic potency compared to chlorpromazine is only 0.2 (1/5). In low doses (in particular 50 to 200 mg daily) its prominent feature is antagonism of presynaptic inhibitory dopamine receptors accounting for some antidepressant activity and a stimulating effect. Therefore, it is in these doses used as a second line antidepressant. Additionally, it alleviates vertigo.
The benzamide neuroleptics (including sulpiride, amisulpride, and sultopride) have been shown to activate the endogenous gamma-hydroxybutyrate receptor in vivo at therapeutic concentrations.[24] Sulpiride was found in one study in rats to upregulate GHB receptors.[25] GHB has neuroleptic properties and it is believed binding to this receptor may contribute to the effects of these neuroleptics.
Sulpiride, along with clozapine, has been found to activate DNA demethylation in the brain.[26]
| Protein | Binding affinity (Ki [nM]) towards cloned human receptors[27] |
|---|---|
| 5-HT1A | >10000 |
| D1 | >10000 |
| D2 | 9.8 |
| D3 | 8.05 |
| D4 | 54 |
| V3 | >10000 |
History
Sulpiride discovered as a result of a research program by Justin-Besançon and C. Laville at Laboratoires Delagrange who were working to improve the anti-dysrhythmic properties of procainamide; the program led first to metoclopramide and later to sulpiride.[28][29] Laboratoires Delagrange was acquired by Synthelabo in 1991[30][31] which eventually became part of Sanofi.[32]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 "Sulpiride Tablets 200mg, 400mg (SPC)". electronic Medicines Compendium (eMC). Sanofi. 21 January 2010. Retrieved 19 October 2013.
- 1 2 Bressolle, F; Brès, J; Fauré-Jeantis, A (January 1992). "Absolute bioavailability, rate of absorption, and dose proportionality of sulpiride in humans". Journal of Pharmaceutical Sciences. 81 (1): 26–32. doi:10.1002/jps.2600810106. PMID 1619566.
- ↑ Imondi, AR; Alam, AS; Brennan, JJ; Hagerman, LM (March 1979). "Metabolism of sulpiride in man and Rhesus monkey". Archives Internationales de Pharmacodynamie et de Thérapie. 232 (1): 79–91. PMID 96745.
- ↑ Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- 1 2 Taylor, D; Paton, C; Shitij, K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97948-8.
- ↑ Wang, J; Omori, IM; Fenton, M; Soares, B (January 2010). "Sulpiride augmentation for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD008125. doi:10.1002/14651858.CD008125.pub2. PMID 20091661.
- ↑ Chia-Cheng Lai, E; Chang, CH; Yang, YHK; Lin, SJ; Lin, CY (2013). "Effectiveness of Sulpiride in Adult Patients With Schizophrenia". Schizophrenia Bulletin. 39 (3): 673–683. doi:10.1093/schbul/sbs002.
- ↑ Soares, BG; Fenton, M; Chue, P (2000). "Sulpiride for schizophrenia". The Cochrane Database of Systematic Reviews (2): CD001162. doi:10.1002/14651858.CD001162. PMID 10796605.
- ↑ Omori, IM; Wang, J; Soares, B; Fenton, M (October 2009). "Sulpiride versus other antipsychotics for schizophrenia (Protocol)". The Cochrane Database of Systematic Reviews (4): CD008126. doi:10.1002/14651858.CD008126.
- ↑ Pani, L; Gessa, GL (2002). "The substituted benzamides and their clinical potential on dysthymia and on the negative symptoms of schizophrenia" (PDF). Molecular Psychiatry. 7 (3): 247–253. doi:10.1038/sj.mp.4001040. PMID 11920152.
- ↑ Uchida, H; Takeuchi, H; Suzuki, T; Nomura, K; Watanabe, K; Kashima, H (December 2005). "Combined treatment with sulpiride and paroxetine for accelerated response in patients with major depressive disorder". Journal of Clinical Psychopharmacology. 25 (6): 545–551. doi:10.1097/01.jcp.0000185425.00644.41. PMID 16282835.
- ↑ Bell, C; Bhikha, S; Colhoun, H; Carter, F; Frampton, C; Porter, R (February 2013). "The response to sulpiride in social anxiety disorder: D2 receptor function". Journal of Psychopharmacology. 27 (2): 146–151. doi:10.1177/0269881112450778. PMID 22745189.
- ↑ Nunes, EA; Freire, RC; Dos Reis, M; de Oliveira, E; Silva, AC; Machado, S; Crippa, JA; Dursun, SM; Baker, GB; Hallak, JE; Nardi, AE (September 2012). "Sulpiride and refractory panic disorder". Psychopharmacology. 223 (2): 247–249. doi:10.1007/s00213-012-2818-6. PMID 22864966.
- ↑ Lepola, U; Koskinen, T; Rimón, R; Salo, H; Gordin, A (July 1989). "Sulpiride and perphenazine in schizophrenia. A double-blind clinical trial". Acta Psychiatrica Scandinavica. 80 (1): 92–96. doi:10.1111/j.1600-0447.1989.tb01305.x. PMID 2669445.
- ↑ Munk-Andersen, E; Behnke, K; Heltberg, J; Nielsen, H; Gerlach, J (1984). "Sulpiride versus haloperidol, a clinical trial in schizophrenia. A preliminary report". Acta Psychiatrica Scandinavica Supplementum. 311: 31–41. PMID 6367362.
- ↑ Gerlach, J; Behnke, K; Heltberg, J; Munk-Anderson, E; Nielsen, H (Sep 1985). "Sulpiride and haloperidol in schizophrenia: a double-blind cross-over study of therapeutic effect, side effects and plasma concentrations". British Journal of Psychiatry. 147: 283–288. doi:10.1192/bjp.147.3.283. PMID 3904885.
- ↑ Standish-Barry, HM; Bouras, N; Bridges, PK; Watson, JP (1983). "A randomized double blind group comparative study of sulpiride and amitriptyline in affective disorder". Psychopharmacology. 81 (3): 258–260. doi:10.1007/bf00427274. PMID 6417717.
- ↑ Quinn, N; Marsden, CD (August 1984). "A double blind trial of sulpiride in Huntington's disease and tardive dyskinesia" (PDF). Journal of Neurology, Neurosurgery and Psychiatry. 47 (8): 844–847. doi:10.1136/jnnp.47.8.844. PMC 1027949
 . PMID 6236286.
. PMID 6236286. - ↑ Peselow, ED; Stanley, M (1982). "Clinical trials of benzamides in psychiatry". Advances in Biochemical Psychopharmacology. 35: 163–194. PMID 6756060.
- ↑ Edwards, JG; Alexander, JR; Alexander, MS; Gordon, A; Zutchi, T (December 1980). "Controlled trial of sulpiride in chronic schizophrenic patients". The British Journal of Psychiatry. 137: 522–529. doi:10.1192/bjp.137.6.522. PMID 7011469.
- ↑ Levkovitz, H; Abramovitch, Y; Nitzan, I (June 1994). "Leukocytosis related to the therapeutic dosage of sulpiride". Biological Psychiatry. 35 (12): 963. doi:10.1016/0006-3223(94)91244-0. PMID 8080896.
- ↑ Melzer, E; Knobel, B (December 1987). "Severe cholestatic jaundice due to sulpiride". Israel Journal of Medical Sciences. 23 (12): 1259–1260. PMID 3326861.
- ↑ Ohmoto, K; Yamamoto, S; Hirokawa, M (December 1999). "Symptomatic primary biliary cirrhosis triggered by administration of sulpiride". The American Journal of Gastroenterology. 94 (12): 3660–3661. doi:10.1111/j.1572-0241.1999.01634.x. PMID 10606349.
- ↑ Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V (Apr 1994). "Displacement of [3H] gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics". Eur J Pharmacol. 256 (2): 211–4. doi:10.1016/0014-2999(94)90248-8. PMID 7914168.
- ↑ Ratomponirina C, Gobaille S, Hodé Y, Kemmel V, Maitre M (Apr 1998). "Sulpiride, but not haloperidol, up-regulates gamma-hydroxybutyrate receptors in vivo and in cultured cells". Eur J Pharmacol. 346 (2–3): 331–7. doi:10.1016/S0014-2999(98)00068-5. PMID 9652377.
- ↑ Dong, E; Nelson, M; Grayson, DR; Costa, E; Guidotti, A (August 2008). "Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 105 (36): 13614–13619. doi:10.1073/pnas.0805493105. PMC 2533238
 . PMID 18757738.
. PMID 18757738. - ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 19 October 2013.
- ↑ Walter Sneader (31 October 2005). Drug Discovery: A History. John Wiley & Sons. pp. 205–. ISBN 978-0-470-01552-0.
- ↑ Sanger GJ Translating 5-HT receptor pharmacology. Neurogastroenterol Motil. 2009 Dec;21(12):1235-8. PMID 19906028 Free full text
- ↑ Denis Conard for Les Echos. Oct 17, 1991 Synthélabo rachète les laboratoires Delagrange
- ↑ Bibliothèque nationale de France Laboratoires Delagrange Page accessed Aug 24, 2016
- ↑ Tom Meek for PMLiVE May 24, 2013 A look back at Sanofi's merger with Synthélabo