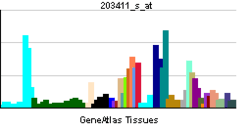
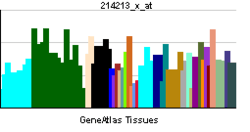
LMNA
| View/Edit Human | View/Edit Mouse |
Lamin A/C also known as LMNA is a protein that in humans is encoded by the LMNA gene.[3][4] Lamin A/C belongs to the lamin family of proteins.
Function
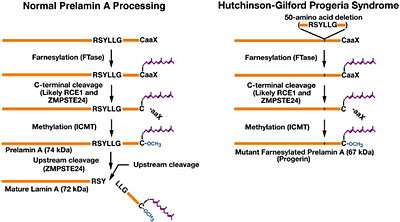
In the setting of ZMPSTE24 deficiency, the final step of lamin processing does not occur, resulting in an accumulation of farnesyl-prelamin A. In Hutchinson–Gilford progeria syndrome, a 50-amino acid deletion in prelamin A (amino acids 607–656) removes the site for the second endoproteolytic cleavage. Consequently, no mature lamin A is formed, and a farnesylated mutant prelamin A (progerin) accumulates in cells.[5] The nuclear lamina consist of a two-dimensional matrix of proteins located next to the inner nuclear membrane. The lamin family of proteins make up the matrix and are highly conserved in evolution. During mitosis, the lamina matrix is reversibly disassembled as the lamin proteins are phosphorylated. Lamin proteins are thought to be involved in nuclear stability, chromatin structure and gene expression. Vertebrate lamins consist of two types, A and B. Through alternate splicing, this gene encodes three type A lamin isoforms.[6]
Early in mitosis, maturation promoting factor (abbreviated MPF, also called mitosis-promoting factor or M-phase-promoting factor) phosphorylates specific serine residues in all three nuclear lamins, causing depolymerization of the lamin intermediate filaments. The phosphorylated lamin B dimers remain associated with the nuclear membrane via their isoprenyl anchor. Lamin A is targeted to the nuclear membrane by an isoprenyl group but it is cleaved shortly after arriving at the membrane. It stays associated with the membrane through protein-protein interactions of itself and other membrane associated proteins, such as LAP1. Depolymerization of the nuclear lamins leads to disintegration of the nuclear envelope. Transfection experiments demonstrate that phosphorylation of human lamin A is required for lamin depolymerization, and thus for disassembly of the nuclear envelope, which normally occurs early in mitosis.
Clinical significance
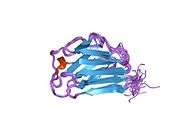
_mutation_R527L_PMID_22549407_surface_and_cartoon.png)
Mutations in the LMNA gene are associated with several diseases, including Emery–Dreifuss muscular dystrophy, familial partial lipodystrophy, limb girdle muscular dystrophy, dilated cardiomyopathy, Charcot–Marie–Tooth disease, restrictive dermopathy and Hutchinson–Gilford progeria syndrome. A truncated version of lamin A, commonly known as progerin, causes Hutchinson–Gilford progeria syndrome.[8][9] To date over 1,400 SNPs are known . They can manifest in changes on mRNA, splicing or protein (e.g. Arg471Cys,[10] Arg482Gln,[11] Arg527Leu,[12] Arg527Cys,[13] Ala529Val [14] ) level.
Interactions
LMNA has been shown to interact with:
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Kamat AK, Rocchi M, Smith DI, Miller OJ (March 1993). "Lamin A/C gene and a related sequence map to human chromosomes 1q12.1-q23 and 10". Somat. Cell Mol. Genet. 19 (2): 203–8. doi:10.1007/BF01233534. PMID 8511676.
- ↑ Wydner KL, McNeil JA, Lin F, Worman HJ, Lawrence JB (March 1996). "Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization". Genomics. 32 (3): 474–8. doi:10.1006/geno.1996.0146. PMID 8838815.
- ↑ Coutinho HD, Falcão-Silva VS, Gonçalves GF, da Nóbrega RB (2009). "Molecular ageing in progeroid syndromes: Hutchinson–Gilford progeria syndrome as a model". Immun Ageing. 6: 4. doi:10.1186/1742-4933-6-4. PMC 2674425
 . PMID 19379495.
. PMID 19379495. - ↑ "Entrez Gene: LMNA lamin A/C".
- ↑ Al-Haggar M, Madej-Pilarczyk A, Kozlowski L, Bujnicki JM, Yahia S, Abdel-Hadi D, Shams A, Ahmad N, Hamed S, Puzianowska-Kuznicka M (2012). "A novel homozygous p.Arg527Leu LMNA mutation in two unrelated Egyptian families causes overlapping mandibuloacral dysplasia and progeria syndrome". Eur J Hum Genet. 20 (11): 1134–40. doi:10.1038/ejhg.2012.77. PMC 3476705
 . PMID 22549407.
. PMID 22549407. - ↑ Capell BC, Collins FS (December 2006). "Human laminopathies: nuclei gone genetically awry". Nat. Rev. Genet. 7 (12): 940–52. doi:10.1038/nrg1906. PMID 17139325.
- ↑ Rankin J, Ellard S (October 2006). "The laminopathies: a clinical review". Clin. Genet. 70 (4): 261–74. doi:10.1111/j.1399-0004.2006.00677.x. PMID 16965317.
- ↑ Zirn B, Kress W, Grimm T, Berthold LD, Neubauer B, Kuchelmeister K, Müller U, Hahn A (2008). "Association of homozygous LMNA mutation R471C with new phenotype: mandibuloacral dysplasia, progeria, and rigid spine muscular dystrophy". Am J Med Genet A. 146A (8): 1049–1054. doi:10.1002/ajmg.a.32259. PMID 18348272.
- ↑ Cao H, Hegele RA (2002). "Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy". Hum. Molec. Genet. 9 (1): 109–12. doi:10.1093/hmg/9.1.109. PMID 10587585.
- ↑ Al-Haggar M, Madej-Pilarczyk A, Kozlowski L, Bujnicki JM, Yahia S, Abdel-Hadi D, Shams A, Ahmad N, Hamed S, Puzianowska-Kuznicka M (2012). "A novel homozygous p.Arg527Leu LMNA mutation in two unrelated Egyptian families causes overlapping mandibuloacral dysplasia and progeria syndrome". Eur J Hum Genet. 20 (11): 1134–40. doi:10.1038/ejhg.2012.77. PMC 3476705
 . PMID 22549407.
. PMID 22549407. - ↑ Agarwal AK, Kazachkova I, Ten S, Garg A (2008). "Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation". J Clin Endocrinol Metab. 93 (12): 4617–4623. doi:10.1210/jc.2008-0123. PMC 2626450
 . PMID 18796515.
. PMID 18796515. - ↑ Garg A, Cogulu O, Ozkinay F, Onay H, Agarwal AK (2005). "A novel homozygous Ala529Val LMNA mutation in Turkish patients with mandibuloacral dysplasia". J. Clin. Endocrinol. Metab. 90 (9): 5259–64. doi:10.1210/jc.2004-2560. PMID 15998779.
- ↑ Tang K, Finley RL, Nie D, Honn KV (March 2000). "Identification of 12-lipoxygenase interaction with cellular proteins by yeast two-hybrid screening". Biochemistry. 39 (12): 3185–91. doi:10.1021/bi992664v. PMID 10727209.
- ↑ Wilkinson FL, Holaska JM, Zhang Z, Sharma A, Manilal S, Holt I, Stamm S, Wilson KL, Morris GE (June 2003). "Emerin interacts in vitro with the splicing-associated factor, YT521-B". Eur. J. Biochem. 270 (11): 2459–66. doi:10.1046/j.1432-1033.2003.03617.x. PMID 12755701.
- ↑ Lattanzi G, Cenni V, Marmiroli S, Capanni C, Mattioli E, Merlini L, Squarzoni S, Maraldi NM (April 2003). "Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts". Biochem. Biophys. Res. Commun. 303 (3): 764–70. doi:10.1016/S0006-291X(03)00415-7. PMID 12670476.
- ↑ Sakaki M, Koike H, Takahashi N, Sasagawa N, Tomioka S, Arahata K, Ishiura S (February 2001). "Interaction between emerin and nuclear lamins". J. Biochem. 129 (2): 321–7. doi:10.1093/oxfordjournals.jbchem.a002860. PMID 11173535.
- ↑ Clements L, Manilal S, Love DR, Morris GE (January 2000). "Direct interaction between emerin and lamin A". Biochem. Biophys. Res. Commun. 267 (3): 709–14. doi:10.1006/bbrc.1999.2023. PMID 10673356.
- ↑ Barton RM, Worman HJ (October 1999). "Prenylated prelamin A interacts with Narf, a novel nuclear protein". J. Biol. Chem. 274 (42): 30008–18. doi:10.1074/jbc.274.42.30008. PMID 10514485.
- ↑ Lloyd DJ, Trembath RC, Shackleton S (April 2002). "A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies". Hum. Mol. Genet. 11 (7): 769–77. doi:10.1093/hmg/11.7.769. PMID 11929849.
- ↑ Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ (December 2002). "Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein". Mol. Biol. Cell. 13 (12): 4401–13. doi:10.1091/mbc.E02-07-0450. PMC 138642
 . PMID 12475961.
. PMID 12475961. - ↑ Dechat T, Korbei B, Vaughan OA, Vlcek S, Hutchison CJ, Foisner R (October 2000). "Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins". J. Cell. Sci. 113 (19): 3473–84. PMID 10984438.
- ↑ Dreuillet C, Tillit J, Kress M, Ernoult-Lange M (November 2002). "In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C". Nucleic Acids Res. 30 (21): 4634–42. doi:10.1093/nar/gkf587. PMC 135794
 . PMID 12409453.
. PMID 12409453. - ↑ Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, Kaeberlein M, Tryggvason K, Zhou Z (2012). "Resveratrol Rescues SIRT1-Dependent Adult Stem Cell Decline and Alleviates Progeroid Features in Laminopathy-Based Progeria". Cell Metabolism. 16 (6): 738–750. doi:10.1016/j.cmet.2012.11.007. PMID 23217256.
Further reading
- Gruenbaum Y, Wilson KL, Harel A, Goldberg M, Cohen M (2000). "Review: nuclear lamins--structural proteins with fundamental functions". J. Struct. Biol. 129 (2–3): 313–23. doi:10.1006/jsbi.2000.4216. PMID 10806082.
- Worman HJ, Courvalin JC (2000). "The inner nuclear membrane". J. Membr. Biol. 177 (1): 1–11. doi:10.1007/s002320001096. PMID 10960149.
- Burke B, Mounkes LC, Stewart CL (2002). "The nuclear envelope in muscular dystrophy and cardiovascular diseases". Traffic. 2 (10): 675–83. doi:10.1034/j.1600-0854.2001.21001.x. PMID 11576443.
- Mounkes LC, Burke B, Stewart CL (2001). "The A-type lamins: nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases". Trends Cardiovasc. Med. 11 (7): 280–5. doi:10.1016/S1050-1738(01)00126-8. PMID 11709282.
- Vigouroux C, Magré J, Desbois-Mouthon C, Lascols O, Cherqui G, Caron M, Capeau J (2002). "[Major insulin resistance syndromes: clinical and physiopathological aspects]". J. Soc. Biol. 195 (3): 249–57. PMID 11833462.
- Helbling-Leclerc A, Bonne G, Schwartz K (2002). "Emery–Dreifuss muscular dystrophy". Eur. J. Hum. Genet. 10 (3): 157–61. doi:10.1038/sj.ejhg.5200744. PMID 11973618.
- Burke B, Stewart CL (2002). "Life at the edge: the nuclear envelope and human disease". Nat. Rev. Mol. Cell Biol. 3 (8): 575–85. doi:10.1038/nrm879. PMID 12154369.
- Novelli G, D'Apice MR (2004). "The strange case of the "lumper" lamin A/C gene and human premature ageing". Trends in molecular medicine. 9 (9): 370–5. doi:10.1016/S1471-4914(03)00162-X. PMID 13129702.
- Pasotti M, Repetto A, Pisani A, Arbustini E (2004). "[Diseases associated with lamin A/C gene defects: what the clinical cardiologist ought to know]". Italian Heart Journal Supplement. 5 (2): 98–111. PMID 15080529.
- Al-Shali KZ, Hegele RA (2005). "Laminopathies and atherosclerosis". Arterioscler. Thromb. Vasc. Biol. 24 (9): 1591–5. doi:10.1161/01.ATV.0000136392.59656.8b. PMID 15205220.
- Garg A, Cogulu O, Ozkinay F, Onay H, Agarwal AK (2005). "A novel homozygous Ala529Val LMNA mutation in Turkish patients with mandibuloacral dysplasia". J. Clin. Endocrinol. Metab. 90 (9): 5259–64. doi:10.1210/jc.2004-2560. PMID 15998779.
- Lees-Miller SP (2006). "Dysfunction of lamin A triggers a DNA damage response and cellular senescence". DNA Repair (Amst.). 5 (2): 286–9. doi:10.1016/j.dnarep.2005.10.007. PMID 16344005.
- Donadille B, Lascols O, Capeau J, Vigouroux C (2006). "Etiological investigations in apparent type 2 diabetes: when to search for lamin A/C mutations?". Diabetes Metab. 31 (6): 527–32. doi:10.1016/S1262-3636(07)70227-6. PMID 16357800.
- Young SG, Meta M, Yang SH, Fong LG (2007). "Prelamin A farnesylation and progeroid syndromes". J. Biol. Chem. 281 (52): 39741–5. doi:10.1074/jbc.R600033200. PMID 17090536.
- Halaschek-Wiener J, Brooks-Wilson A (2007). "Progeria of stem cells: stem cell exhaustion in Hutchinson–Gilford progeria syndrome". J. Gerontol. A Biol. Sci. Med. Sci. 62 (1): 3–8. doi:10.1093/gerona/62.1.3. PMID 17301031.
- Mazereeuw-Hautier J, Wilson LC, Mohammed S, Smallwood D, Shackleton S, Atherton DJ, Harper JI (2007). "Hutchinson–Gilford progeria syndrome: clinical findings in three patients carrying the G608G mutation in LMNA and review of the literature". Br. J. Dermatol. 156 (6): 1308–14. doi:10.1111/j.1365-2133.2007.07897.x. PMID 17459035.
- Sliwińska MA (2007). "[The role of lamins and mutations of LMNA gene in physiological and premature aging] Polish". Postepy Biochem. 53 (1): 46–52. PMID 17718387.
- Genschel J, Schmidt HH (December 2000). "Mutations in the LMNA gene encoding lamin A/C". Hum. Mutat. 16 (6): 451–9. doi:10.1002/1098-1004(200012)16:6<451::AID-HUMU1>3.0.CO;2-9. PMID 11102973.
- Scaffidi P, Misteli T (April 2005). "Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome". Nat Med. 11 (4): 440–5. doi:10.1038/nm1204. PMC 1351119
 . PMID 15750600.
. PMID 15750600. - "Charcot–Marie–Tooth Neuropathy Type 2". 1993. PMID 20301462.
- "Congenital Muscular Dystrophy Overview". 1993. PMID 20301468.
- "LMNA-Related Dilated Cardiomyopathy". 1993. PMID 20301717.
- "Limb-Girdle Muscular Dystrophy Overview". 1993. PMID 20301582.
- "Emery–Dreifuss Muscular Dystrophy". 1993. PMID 20301609.
- "Hutchinson–Gilford Progeria Syndrome". 1993. PMID 20301300.
- "Dense Deposit Disease/Membranoproliferative Glomerulonephritis Type II". 1993. PMID 20301598.
External links
- Online Mendelian Inheritance in Man (OMIM) Cardiomyopathy, Dilated, 1A; CMD1A -115200
- Online Mendelian Inheritance in Man (OMIM) LAMIN A/C; LMNA -150330
- LMNA protein, human at the US National Library of Medicine Medical Subject Headings (MeSH)
- LOVD mutation database: LMNA
- GeneCards for LMNA