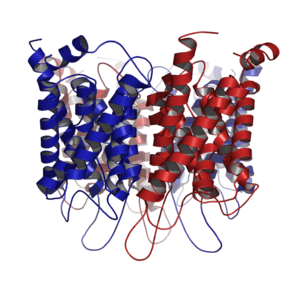
Aquaporin
| Aquaporin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Identifiers | |||||||||
| Symbol | Aquaporin | ||||||||
| Pfam | PF00230 | ||||||||
| InterPro | IPR000425 | ||||||||
| PROSITE | PDOC00193 | ||||||||
| SCOP | 1fx8 | ||||||||
| SUPERFAMILY | 1fx8 | ||||||||
| TCDB | 1.A.8 | ||||||||
| |||||||||
Aquaporins are integral membrane proteins from a larger family of major intrinsic proteins (MIP) that form pores in the membrane of biological cells.[1]
Genetic defects involving aquaporin genes have been associated with several human diseases.[2][3] The 2003 Nobel Prize in Chemistry was awarded jointly to Peter Agre for the discovery of aquaporins,[4] and Roderick MacKinnon for his work on the structure and mechanism of potassium channels.[5] The plasma membranes of a variety of different animal and plant cells contain aquaporins through which water can flow more rapidly inside the cell than by diffusing through the phospholipid bilayer.[6]
Function
Aquaporins are "the plumbing system for cells," said Agre. Every cell is primarily water. "But the water doesn’t just sit in the cell, it moves through it in a very organized way. The process occurs rapidly in tissues that have these aquaporins or water channels."
For many years, scientists assumed that water leaked through the cell membrane, and some water does. "But the very rapid movement of water through some cells was not explained by this theory," said Agre.[7]
Aquaporins selectively conduct water molecules in and out of the cell, while preventing the passage of ions and other solutes. Also known as water channels, aquaporins are integral membrane pore proteins. Some of them, known as aquaglyceroporins, also transport other small uncharged solutes, such as glycerol, CO2, ammonia and urea across the membrane, depending on the size of the pore. For example, the aquaporin 3 channel has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da. However, the water pores are completely impermeable to charged species, such as protons, a property critical for the conservation of the membrane's electrochemical potential difference.[8]
Water molecules traverse through the pore of the channel in single file. The presence of water channels increases membrane permeability to water.
Many human cell types express them, as do certain bacteria and many other organisms, such as plants for which it is essential for the water transport system[9] and tolerance to drought and salt stresses.[10]
Discovery
Agre said he discovered aquaporins "by serendipity." His lab had an N.I.H. grant to study the Rh blood group antigens. They isolated the Rh molecule but a second molecule, 28 kilodaltons in size (and therefore called 28K) kept appearing. At first they thought it was a piece of the Rh molecule, or a contaminant, but it turned out to be an undiscovered molecule with unknown function. It was abundant in red blood cells and kidney tubes, and related to proteins of diverse origins, like the brains of fruit flies, bacteria, the lenses of eyes, and plant tissues.
In most cells, water moves in and out by osmosis through the lipid component of cell membranes. Due to the relatively high water permeability of some epithelial cells it was long suspected that some additional mechanism for water transport across membranes must exist. But it was not until 1992 that the first aquaporin, ‘aquaporin-1’ (originally known as CHIP 28), was reported by Peter Agre, of Johns Hopkins University.[11]
The pioneering discoveries and research on water channels by Agre and his colleagues resulted in the presentation of a Nobel Prize in Chemistry to Agre in 2003.[5] In 1999, together with other research teams, Agre reported the first high-resolution images of the three-dimensional structure of an aquaporin, namely, aquaporin-1.[12] Further studies using supercomputer simulations have identified the pathway of water as it moves through the channel and demonstrated how a pore can allow water to pass without the passage of small solutes.[13] However the first report of protein mediated water transport through membranes was by Gheorghe Benga in 1986.[14][15] This publication that preceded Agre's first publication on water membrane transport proteins has led to a controversy that Benga's work was adequately recognized by neither Agre nor the Nobel Prize Committee.[16] There is a long history of water pores, starting in 1957.[17] There have been many reviews of the history.[18]
Structure


Aquaporin proteins are made up of six transmembrane α-helices arranged in a right-handed bundle, with the amino and the carboxyl termini located on the cytoplasmic surface of the membrane.[8][19] The amino and carboxyl halves of the sequence show similarity to each other, in what appears to be a tandem repeat. Some researchers believe that this results from an early evolution event that saw the duplication of the half-size gene. There are also five interhelical loop regions (A – E) that form the extracellular and cytoplasmic vestibules. Loops B and E are hydrophobic loops that contain the highly, although not completely conserved, asparagine–proline–alanine (NPA) motif, which overlap the middle of the lipid bilayer of the membrane forming a 3-D 'hourglass' structure where the water flows through. This overlap forms one of the two well-known channel constriction sites in the peptide, the NPA motif and a second and usually narrower constriction known as 'selectivity filter' or ar/R selectivity filter.
Aquaporins form tetramers in the cell membrane, with each monomer acting as a water channel.[8] The different aquaporins contain differences in their peptide sequence, which allows for the size of the pore in the protein to differ between aquaporins. The resultant size of the pore directly affects what molecules are able to pass through the pore, with small pore sizes only allowing small molecules like water to pass through the pore.
X-ray profiles show that aquaporins have two conical entrances. This hourglass shape could be the result of a natural selection process toward optimal permeability. It has been shown that conical entrances with suitable opening angle can indeed provide a large increase of the hydrodynamic channel permeability.[20]
NPA motif
Using computer simulations, it has been suggested that the orientation of the water molecules moving through the channel assures that only water passes between cells, due to the formation of a single line of water molecules. The water molecules move through the narrow channel by orienting themselves in the local electrical field formed by the atoms of the channel wall. Upon entering, the water molecules face with their oxygen atom down the channel. Midstream, they reverse orientation, facing with the oxygen atom up.[21]
Why this rotation occurs is not entirely clear yet. Some researchers identified an electrostatic field generated by the two aquaporin half-helices HB and HE as the reason for the rotation of water molecules. Others suggested that it is caused by the interaction of hydrogen bonds between the oxygen of the water molecule and the asparagines in the two NPA motifs. Moreover, whether the rotation of water molecules has any biological significance is still being discussed. Early studies speculated that the "bipolar" orientation of water molecules keep them from conducting protons via the Grotthuss mechanism, while still permitting a fast flux of water molecules.[22] More recent studies question this interpretation and emphasize an electrostatic barrier as the reason for proton blockage. In the latter view, the rotation of water molecules is only a side-effect of the electrostatic barrier. At present (2008), the origin of the electrostatic field is a matter of debate. While some studies mainly considered the electric field generated by the protein's half-helices HB and HE, others emphasized desolvation effects as the proton enters the narrow aquaporin pore.
ar/R selectivity filter

The ar/R (aromatic/arginine) selectivity filter is a cluster of amino acids that help bind to water molecules and exclude other molecules that may try to enter the pore. It is the mechanism by which the aquaporin is able to selectively bind water molecules (hence allowing them through) and prevent other molecules from entering. The ar/R filter is a tetrad that is formed by two amino acid residues from helices B (HB) and E (HE) and two residues from loop E (LE1 and LE2), found on either side of the NPA motif. The ar/R region is usually found towards the extracellular vestibule, approximately 8 Å above the NPA motif and is often the narrowest part of the pore. The narrow pore acts to weaken the hydrogen bonds between the water molecules allowing the water to interact with the positively charged arginine, which also acts as a proton filter for the pore.
Species distribution
In mammals
There are thirteen known types of aquaporins in mammals, and six of these are located in the kidney,[23] but the existence of many more is suspected. The most studied aquaporins are compared in the following table:
| Type | Location[24] | Function[24] |
|---|---|---|
| Aquaporin 1 | Water reabsorption | |
| Aquaporin 2 | Water reabsorption in response to ADH[25] | |
| Aquaporin 3 | Water reabsorption and glycerol permeability | |
| Aquaporin 4 | Water reabsorption |
In plants
In plants water is taken up from the soil through the roots, where it passes from the cortex into the vascular tissues. There are three routes for water to flow in these tissues, known as the apoplastic, symplastic and transcellular pathways.[26] Specifically, aquaporins are found in the vacuolar membrane, in addition to the plasma membrane of plants; the transcellular pathway involves transport of water across the plasma and vacuolar membrane.[27] When plant roots are exposed to mercuric chloride, which is known to inhibit aquaporins, the flow of water is greatly reduced while the flow of ions is not, supporting the view that there exists a mechanism for water transport independent of the transport of ions: aquaporins.
In addition to the maintenance of normal cytosolic osmolarity, aquaporins can play a major role in extension growth by allowing an influx of water into expanding cells - a process necessary to sustain plant development.[27]
Aquaporins in plants are separated into five main homologous subfamilies, or groups:[28]
- Plasma membrane Intrinsic Protein (PIP)[29]
- Tonoplast Intrinsic Protein (TIP)[30]
- Nodulin-26 like Intrinsic Protein (NIP)[31]
- Small basic Intrinsic Protein (SIP)[32]
- X Intrinsic Protein (XIP)
These five subfamilies have later been divided into smaller evolutionary subgroups based on their DNA sequence. PIPs cluster into two subgroups, PIP1 and PIP2, whilst TIPs cluster into 5 subgroups, TIP1, TIP2, TIP3, TIP4 and TIP5. Each subgroup is again split up into isoforms e.g. PIP1;1, PIP1;2. Within the various selection of aquaporin isoforms in plants, there are also unique patterns of cell- and tissue-specific expression.[27]
The silencing of plant aquaporins has been linked to poor plant growth and even death of the plant.
The gating of aquaporins is carried out to stop the flow of water through the pore of the protein. This may be carried out for a number of reasons, for example when the plant contains low amounts of cellular water due to drought.[33] The gating of an aquaporin is carried out by an interaction between a gating mechanism and the aquaporin, which causes a 3D change in the protein so that it blocks the pore and, thus, disallows the flow of water through the pore. In plants, it has been seen that there are at least two forms of aquaporin gating. These are gating by the dephosphorylation of certain serine residues, which has been seen as a response to drought, and the protonation of specific histidine residues in response to flooding. The phosphorylation of an aquaporin has also been linked to the opening and closing of petals in response to temperature.[34][35]
Clinical significance
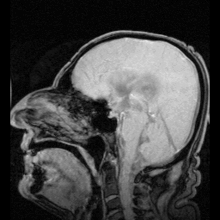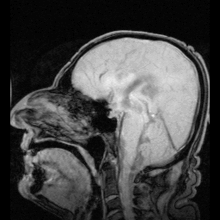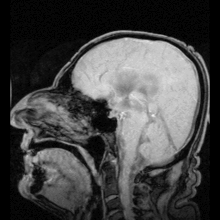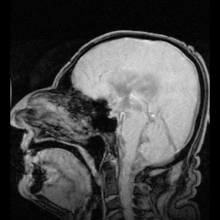
If aquaporin could be manipulated, that could potentially solve medical problems such as fluid retention in heart disease and brain edema after stroke.[7]
There have been two clear examples of diseases identified as resulting from mutations in aquaporins:
- Mutations in the aquaporin-2 gene cause hereditary nephrogenic diabetes insipidus in humans.[36]
- Mice homozygous for inactivating mutations in the aquaporin-0 gene develop congenital cataracts.[37]
A small number of people have been identified with severe or total deficiency in aquaporin-1. It is interesting to note that they are, in general, healthy, but exhibit a defect in the ability to concentrate solutes in the urine and to conserve water when deprived of drinking water. Mice with targeted deletions in aquaporin-1 also exhibit a deficiency in water conservation due to an inability to concentrate solutes in the kidney medulla by countercurrent multiplication.
In addition to its role in genetically determined nephrogenic diabetes insipidus, aquaporins also play a key role in acquired forms of nephrogenic diabetes insipidus (disorders that cause increased urine production).[38] Acquired nephrogenic diabetes insipidus can result from impaired regulation of aquaporin-2 due to administration of lithium salts (as a treatment for bipolar disorder), low potassium concentrations in the blood (hypokalemia), high calcium concentrations in the blood (hypercalcemia), or a chronically high intake of water beyond the normal requirements (e.g., due to excessive habitual intake of bottled water or coffee).
It has been found that autoimmune reactions against aquaporin 4 produce Devic's disease.[39]
Aquaporin from kidneys is influenced by vasopressin.
References
- ↑ Agre P (2006). "The aquaporin water channels". Proc Am Thorac Soc. 3 (1): 5–13. doi:10.1513/pats.200510-109JH. PMC 2658677
 . PMID 16493146.
. PMID 16493146. - ↑ Agre P, Kozono D (2003). "Aquaporin water channels: molecular mechanisms for human diseases". FEBS Lett. 555 (1): 72–8. doi:10.1016/S0014-5793(03)01083-4. PMID 14630322.
- ↑ Schrier RW (2007). "Aquaporin-related disorders of water homeostasis". Drug News Perspect. 20 (7): 447–53. doi:10.1358/dnp.2007.20.7.1138161. PMID 17992267.
- ↑ Knepper MA, Nielsen S (2004). "Peter Agre, 2003 Nobel Prize winner in chemistry". J. Am. Soc. Nephrol. 15 (4): 1093–5. doi:10.1097/01.ASN.0000118814.47663.7D. PMID 15034115.
- 1 2 "The Nobel Prize in Chemistry 2003". Nobel Foundation. Retrieved 2008-01-23.
- ↑ Cooper, Geoffrey (2009). The Cell: A Molecular Approach. Washington, DC: ASM PRESS. p. 544. ISBN 9780878933006.
- 1 2 A Conversation With Peter Agre: Using a Leadership Role to Put a Human Face on Science, By Claudia Dreifus, New YorkTimes, January 26, 2009
- 1 2 3 Gonen T, Walz T (2006). "The structure of aquaporins". Q. Rev. Biophys. 39 (4): 361–96. doi:10.1017/S0033583506004458. PMID 17156589.
- ↑ Kruse E, Uehlein N, Kaldenhoff R (2006). "The aquaporins". Genome Biol. 7 (2): 206. doi:10.1186/gb-2006-7-2-206. PMC 1431727
 . PMID 16522221.
. PMID 16522221. - ↑ Xu Y, et al. (2014). "A banana aquaporin gene". BMC Plant Biology. 14 (1): 59. doi:10.1186/1471-2229-14-59. PMID 24606771.
- ↑ Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S (1 October 1993). "Aquaporin CHIP: the archetypal molecular water channel". Am. J. Physiol. 265 (4 Pt 2): F463–76. PMID 7694481.
- ↑ Mitsuoka K, Murata K, Walz T, Hirai T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (1999). "The structure of aquaporin-1 at 4.5-A resolution reveals short alpha-helices in the center of the monomer". J. Struct. Biol. 128 (1): 34–43. doi:10.1006/jsbi.1999.4177. PMID 10600556.
- ↑ de Groot BL, Grubmüller H (2005). "The dynamics and energetics of water permeation and proton exclusion in aquaporins". Curr. Opin. Struct. Biol. 15 (2): 176–83. doi:10.1016/j.sbi.2005.02.003. PMID 15837176.
- ↑ Benga G, Popescu O, Pop VI, Holmes RP (1986). "p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes". Biochemistry. 25 (7): 1535–8. doi:10.1021/bi00355a011. PMID 3011064.
- ↑ Kuchel PW (2006). "The story of the discovery of aquaporins: convergent evolution of ideas--but who got there first?". Cell. Mol. Biol. (Noisy-le-grand). 52 (7): 2–5. PMID 17543213.
- ↑ G Benga. "Gheorghe Benga". Ad Astra - Online project for the Romanian Scientific Community. Archived from the original on December 25, 2007. Retrieved 2008-04-05.
- ↑ Paganelli CV, Solomon AK (November 1957). "The rate of exchange of tritiated water across the human red cell membrane". J. Gen. Physiol. 41 (2): 259–77. doi:10.1085/jgp.41.2.259. PMC 2194835
 . PMID 13475690.
. PMID 13475690. - ↑ Parisi M, Dorr RA, Ozu M, Toriano R (December 2007). "From membrane pores to aquaporins: 50 years measuring water fluxes". J Biol Phys. 33 (5–6): 331–43. doi:10.1007/s10867-008-9064-5. PMC 2565768
 . PMID 19669522.
. PMID 19669522. - ↑ Fu D, Lu M (2007). "The structural basis of water permeation and proton exclusion in aquaporins". Mol. Membr. Biol. 24 (5–6): 366–74. doi:10.1080/09687680701446965. PMID 17710641.
- ↑ Gravelle S, Joly L, Detcheverry F, Ybert C, Cottin-Bizonne C, Bocquet L (2013). "Optimizing water permeability through the hourglass shape of aquaporins". PNAS. 110 (41): 16367–16372. arXiv:1310.4309
 . Bibcode:2013PNAS..11016367G. doi:10.1073/pnas.1306447110. PMC 3799357
. Bibcode:2013PNAS..11016367G. doi:10.1073/pnas.1306447110. PMC 3799357 . PMID 24067650.
. PMID 24067650. - ↑ de Groot BL, Grubmüller H (2001). "Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF". Science. 294 (5550): 2353–2357. Bibcode:2001Sci...294.2353D. doi:10.1126/science.1062459. PMID 11743202.
- ↑ Tajkhorshid E, Nollert P, Jensen M, Miercke LJ, O'Connell J, Stroud RM, Schulten K (2002). "Control of the selectivity of the aquaporin water channel family by global orientational tuning". Science. 296 (5567): 525–30. Bibcode:2002Sci...296..525T. doi:10.1126/science.1067778. PMID 11964478.
- ↑ Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA (2002). "Aquaporins in the kidney: from molecules to medicine". Physiol. Rev. 82 (1): 205–44. doi:10.1152/physrev.00024.2001 (inactive 2015-01-09). PMID 11773613.
- 1 2 Unless else specified in table boxes, then ref is: Walter F. Boron (2005). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 842
- ↑ Sands JM. Aquaporin 2: Not Just for Moving Water. Journal of the American Society of Nephrology : JASN. 2012;23(9):1443-1444. doi:10.1681/ASN.2012060613.
- ↑ Chaumont, François; Tyerman, Stephen D. (2014-04-01). "Aquaporins: Highly Regulated Channels Controlling Plant Water Relations1". Plant Physiology. 164 (4): 1600–1618. doi:10.1104/pp.113.233791. ISSN 0032-0889. PMC 3982727
 . PMID 24449709.
. PMID 24449709. - 1 2 3 Johansson, Ingela; Karlsson, Maria; Johanson, Urban; Larsson, Christer; Kjellbom, Per (2000-05-01). "The role of aquaporins in cellular and whole plant water balance". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1465 (1–2): 324–342. doi:10.1016/S0005-2736(00)00147-4.
- ↑ Kaldenhoff R, Bertl A, Otto B, Moshelion M, Uehlein N (2007). "Characterization of plant aquaporins". Meth. Enzymol. Methods in Enzymology. 428: 505–31. doi:10.1016/S0076-6879(07)28028-0. ISBN 9780123739216. PMID 17875436.
- ↑ Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994). "Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system". Plant J. 6 (2): 187–99. doi:10.1046/j.1365-313X.1994.6020187.x. PMID 7920711.
- ↑ Maeshima M (2001). "TONOPLAST TRANSPORTERS: Organization and Function". Annu Rev Plant Physiol Plant Mol Biol. 52 (1): 469–497. doi:10.1146/annurev.arplant.52.1.469. PMID 11337406.
- ↑ Wallace IS, Choi WG, Roberts DM (2006). "The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins". Biochim. Biophys. Acta. 1758 (8): 1165–75. doi:10.1016/j.bbamem.2006.03.024. PMID 16716251.
- ↑ Johanson U, Gustavsson S (2002). "A new subfamily of major intrinsic proteins in plants". Mol. Biol. Evol. 19 (4): 456–61. doi:10.1093/oxfordjournals.molbev.a004101. PMID 11919287.
- ↑ Kaldenhoff R, Fischer M (2006). "Aquaporins in plants". Acta Physiol (Oxf). 187 (1–2): 169–76. doi:10.1111/j.1748-1716.2006.01563.x. PMID 16734753.
- ↑ Azad AK, Sawa Y, Ishikawa T, Shibata H (2004). "Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals". Plant Cell Physio. 45(5) (5): 608–17. doi:10.1093/pcp/pch069. PMID 15169943.
- ↑ Azad AK, Katsuhara M, Sawa Y, Ishikawa T, Shibata H (2008). "Characterization of four plasma membrane aquaporins in tulip petals: a putative homolog is regulated by phosphorylation". Plant Cell Physiol. 49(8) (8): 1196–208. doi:10.1093/pcp/pcn095. PMID 18567892.
- ↑ Bichet DG (2006). "Nephrogenic diabetes insipidus". Adv Chronic Kidney Dis. 13 (2): 96–104. doi:10.1053/j.ackd.2006.01.006. PMID 16580609.
- ↑ Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N (2003). "Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice". Genomics. 81 (4): 361–8. doi:10.1016/S0888-7543(03)00029-6. PMID 12676560.
- ↑ Khanna A (2006). "Acquired nephrogenic diabetes insipidus". Semin. Nephrol. 26 (3): 244–8. doi:10.1016/j.semnephrol.2006.03.004. PMID 16713497.
- ↑ Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005). "IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel". J. Exp. Med. 202 (4): 473–7. doi:10.1084/jem.20050304. PMC 2212860
 . PMID 16087714.
. PMID 16087714.
External links
- Aquaporins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Animation (MPEG file at nobel.se)
- Bowen R. "Aquaporins: Water Channels". Colorado State University. Archived from the original on 2 March 2008. Retrieved 2008-01-23.
- Mallery C. "Aquaporins - Water Channels". University of Miami. Retrieved 2008-01-23.
- Harrison N. "Describe how aquaporins enable water to cross cell membranes. Comment on the physiological roles of AQP and of related transporters". Retrieved 2008-01-23.
- Computational Biomolecular Dynamics Group. "Aquaporin movies and pictures". Max Planck Institute. Archived from the original on April 25, 2006. Retrieved 2008-01-23.
- Orientation of Proteins in Membranes Group. "Aquaporin movies and pictures: Calculated positions of aquaporins of known 3D structure in membrane". University of Michigan. Archived from the original on 25 February 2008. Retrieved 2008-01-23.
- Theoretical and Computational Biophysics Group. "Structure, Dynamics, and Function of Aquaporins". University of Illinois at Urbana-Champaign. Retrieved 2008-01-23.
- Artem B. Mamonov; Rob D. Coalson; Mark L. Zeidel; John C. Mathai. "Water and Deuterium Oxide Permeability through Aquaporin 1: MD Predictions and Experimental Verification". Journal of General Physiology. Retrieved 2014-09-19.