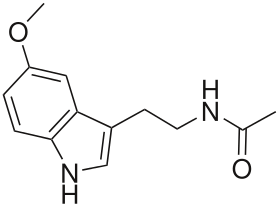
Melatonin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation |
|
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | Oral, sublingual, transdermal |
| ATC code | N05CH01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30–50% |
| Metabolism | Hepatic via CYP1A2 mediated 6-hydroxylation |
| Biological half-life | 35–50 minutes |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
73-31-4 |
| PubChem (CID) | 896 |
| IUPHAR/BPS | 224 |
| DrugBank |
DB01065 |
| ChemSpider |
872 |
| UNII |
JL5DK93RCL |
| KEGG |
D08170 |
| ChEBI |
CHEBI:16796 |
| ChEMBL |
CHEMBL45 |
| Chemical and physical data | |
| Formula | C13H16N2O2 |
| Molar mass | 232.278 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Melatonin also known as N-acetyl-5-methoxy tryptamine,[1] is a hormone that is produced by the pineal gland in animals that regulates sleep and wakefulness.[2] Melatonin is also produced in plants where is functions as a first line of defense against oxidative stress.[3]
In animals, melatonin is involved in the entrainment (synchronization) of the circadian rhythms of physiological functions including sleep timing, blood pressure regulation, seasonal reproduction, and many others.[4] Many of melatonin's biological effects in animals are produced through activation of melatonin receptors,[5] while others are due to its role as an antioxidant,[6] with a particular role in the protection of nuclear and mitochondrial DNA.[7]
It is used as a medication for insomnia, however, scientific evidence is insufficient to demonstrate a benefit in this area.[8] Melatonin is sold over-the-counter in the United States and Canada. In other countries, it may require a prescription or it may be unavailable.
Medical uses

Sleep disorders
Melatonin has shown promise in treating sleep-wake cycle disorders in children with underlying neurodevelopment difficulties.[9][10] As add-on to antihypertensive therapy, prolonged-release melatonin has improved blood pressure control in people with nocturnal hypertension.[11]
People with circadian rhythm sleep disorders may use oral melatonin to help entrain (biologically synchronize in the correct phase) to the environmental light-dark cycle. Melatonin reduces sleep onset latency to a greater extent in people with delayed sleep phase disorder than in people with insomnia.[12]
Melatonin has been studied for insomnia in the elderly.[13][14][15] Prolonged-release melatonin has shown good results in treating insomnia in older adults.[16] Short-term treatment (up to three months) of prolonged-release melatonin was found to be effective and safe in improving sleep latency, sleep quality, and daytime alertness.[17]
Evidence for use of melatonin as a treatment for insomnia is, as of 2015, insufficient;[8] low-quality evidence indicates it may speed the onset of sleep by 6 minutes.[8] A 2004 review found "no evidence that melatonin had an effect on sleep onset latency or sleep efficiency" in shift work or jet lag, while it did decrease sleep onset latency in people with a primary sleep disorder and it increased sleep efficiency in people with a secondary sleep disorder.[12] A later review[18] found minimal evidence for efficacy in shift work.
Jet lag and shift work
Melatonin is known to aid in reducing the effects of jet lag, especially in eastward travel, by promoting the necessary reset of the body's sleep-wake phase. If the timing is not correct, however, it can instead delay adaption.[19]
Melatonin appears also to have limited use against the sleep problems of people who work rotating or night shifts.[18]
Headaches
Tentative evidence shows melatonin may help reduce some types of headaches including cluster headaches.[20]
Cancer
A 2013 review by the National Cancer Institutes found evidence for use to be inconclusive.[21] A 2005 review of unblinded clinical trials found a reduced rate of death, but that blinded and independently conducted randomized controlled trials are needed.[22]
Gallstones
Melatonin presence in the gallbladder has many protective properties, such as converting cholesterol to bile, preventing oxidative stress, and increasing the mobility of gallstones from the gallbladder.[23]
Protection from radiation
Both animal[24] and human[25][26] studies have shown melatonin to protect against radiation-induced cellular damage. Melatonin and its metabolites protect organisms from oxidative stress by scavenging reactive oxygen species which are generated during exposure.[27] Nearly 70% of biological damage caused by ionizing radiation is estimated to be attributable to the creation of free radicals, especially the hydroxyl radical that attacks DNA, proteins, and cellular membranes. Melatonin has been described as a broadly protective, readily available, and orally self-administered antioxidant that is without major known side effects.[28]
Tinnitus
Tentative evidence of benefit exists for treating tinnitus.[29]
Psychiatry
Melatonin might improve sleep in autistic people.[30] Children with autism have abnormal melatonin pathways and below-average physiological levels of melatonin.[31][32] Melatonin supplementation has been shown to improve sleep duration, sleep onset latency, and night-time awakenings.[31][33][34] However, many studies on melatonin and autism rely on self-reported levels of improvement and more rigorous research is needed.
While the packaging of melatonin often warns against use in people under 18 years of age, available studies suggest that melatonin is an efficacious and safe treatment for insomnia in people with ADHD. However, larger and longer studies are needed to establish long-term safety and optimal dosing.[35]
Melatonin in comparison to placebo is effective for reducing preoperative anxiety in adults when given as premedication. It may be just as effective as standard treatment with midazolam in reducing preoperative anxiety. Melatonin may also reduce postoperative anxiety (measured 6 hours after surgery) when compared to placebo.[36]
Some supplemental melatonin users report an increase in vivid dreaming. Extremely high doses of melatonin increased REM sleep time and dream activity in people both with and without narcolepsy.[37]
Adverse effects
Melatonin appears to cause very few side effects as tested in the short term, up to three months, at low doses. Two systematic reviews found no adverse effects of exogenous melatonin in several clinical trials and comparative trials found the adverse effects headaches, dizziness, nausea, and drowsiness were reported about equally for both melatonin and placebo.[38][39] Prolonged-release melatonin is safe with long-term use of up to 12 months.[40]
Melatonin can cause nausea, next-day grogginess, and irritability.[41] In the elderly, it can cause reduced blood flow and hypothermia.[42] In autoimmune disorders, evidence is conflicting whether melatonin supplementation may ameliorate or exacerbate symptoms due to immunomodulation.[43][44]
Melatonin can lower follicle-stimulating hormone levels.[45] Effects of melatonin on human reproduction remain unclear,[46] although it was with some effect tried as a contraceptive in the 1990s.[47]
Anticoagulants and other substances are known to interact with melatonin.[48]
Functions
Circadian rhythm
In animals, the primary function is regulation of day-night cycles. Human infants' melatonin levels become regular in about the third month after birth, with the highest levels measured between midnight and 8:00 am.[49] Human melatonin production decreases as a person ages.[50] Also, as children become teenagers, the nightly schedule of melatonin release is delayed, leading to later sleeping and waking times.[51]
Antioxidant
Besides its function as synchronizer of the biological clock, melatonin is a powerful free-radical scavenger and wide-spectrum antioxidant as discovered in 1993.[52] In many less-complex life forms, this is its only known function.[27] Melatonin is an antioxidant that can easily cross cell membranes and the blood–brain barrier.[6][53] This antioxidant is a direct scavenger of radical oxygen and nitrogen species including OH•, O•2−, and NO•.[54][55] Melatonin works with other antioxidants to improve the overall effectiveness of each antioxidant.[55] Melatonin has been proven to be twice as active as vitamin E, believed to be the most effective lipophilic antioxidant.[56] An important characteristic of melatonin that distinguishes it from other classic radical scavengers is that its metabolites are also scavengers in what is referred to as the cascade reaction.[27] Also different from other classic antioxidants, such as vitamin C and vitamin E, melatonin has amphiphilic properties. When compared to synthetic, mitochondrial-targeted antioxidants (MitoQ and MitoE), melatonin proved to be a comparable protector against mitochondrial oxidative stress.[57]
Immune system
While it is known that melatonin interacts with the immune system,[58][59] the details of those interactions are unclear. Antiinflammatory effect seems to be the most relevant and most documented in the literature. There have been few trials designed to judge the effectiveness of melatonin in disease treatment. Most existing data are based on small, incomplete clinical trials. Any positive immunological effect is thought to be the result of melatonin acting on high-affinity receptors (MT1 and MT2) expressed in immunocompetent cells. In preclinical studies, melatonin may enhance cytokine production,[60] and by doing this, counteract acquired immunodeficiences. Some studies also suggest that melatonin might be useful fighting infectious disease[61] including viral, such as HIV, and bacterial infections, and potentially in the treatment of cancer.
Metal chelation
In vitro, melatonin can form complexes with cadmium and other metals.[62]
Biosynthesis and pharmacology
Biosynthesis

In animals, biosynthesis of melatonin occurs through hydroxylation, decarboxylation, acetylation and a methylation starting with L-tryptophan.[63] L-tryptophan is produced in the shikimate pathway from chorismate or is acquired from protein catabolism. First L-tryptophan is hydroxylated on the indole ring by tryptophan hydroxylase to produce 5-hydroxytryptophan. This intermediate (5-HTP) is decarboxylated by Pyridoxal phosphate and 5-hydroxytryptophan decarboxylase to produce serotonin. Serotonin is itself an important neurotransmitter, but is also converted into N-Acetylserotonin by serotonin N-acetyltransferase and acetyl-CoA. Hydroxyindole O-methyltransferase and S-Adenosyl methionine convert N-acetylserotonin into melatonin through methylation of the hydroxyl group.
In bacteria, protists, fungi, and plants, melatonin is synthesized indirectly with tryptophan as an intermediate product of the shikimate pathway. In these cells, synthesis starts with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells with carbon dioxide. The rest of the synthesising reactions are similar, but with slight variations in the last two enzymes.[64][65]
Mechanism

In order to hydroxylate L-tryptophan, the cofactor tetrahydrobiopterin (THB) must first react with oxygen and the active site iron of tryptophan hydroxylase. This mechanism is not well understood, but two mechanisms have been proposed:
1. A slow transfer of one electron from the THB to O2 could produce a superoxide which could recombine with the THB radical to give 4a-peroxypterin. 4a-peroxypterin could then react with the active site iron (II) to form an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron.
2. O2 could react with the active site iron (II) first, producing iron (III) superoxide which could then react with the THB to form an iron-peroxypterin intermediate.
Iron (IV) oxide from the iron-peroxypterin intermediate is selectively attacked by a double bond to give a carbocation at the C5 position of the indole ring. A 1,2-shift of the hydrogen and then a loss of one of the two hydrogen atoms on C5 reestablishes aromaticity to furnish 5-hydroxy-L-tryptophan.[66]
A decarboxylase with cofactor pyridoxal phosphate (PLP) removes CO2 from 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine.[67] PLP forms an imine with the amino acid derivative. The amine on the pyridine is protonated and acts as an electron sink, enabling the breaking of the C-C bond and releasing CO2. Protonation of the amine from tryptophan restores the aromaticity of the pyridine ring and then imine is hydrolyzed to produce 5-hydroxytryptamine and PLP.[68]
It has been proposed that histidine residue His122 of serotonin N-acetyl transferase is the catalytic residue that deprotonates the primary amine of 5-hydroxytryptamine, which allows the lone pair on the amine to attack acetyl-CoA, forming a tetraherdral intermediate. The thiol from coenzyme A serves as a good leaving group when attacked by a general base to give N-acetylserotonin.[69]
N-acetylserotonin is methylated at the hydroxyl position by S-adenosyl methionine (SAM) to produce S-adenosyl homocysteine (SAH) and melatonin.[70][71]
Regulation
In vertebrates, melatonin secretion is regulated by norepinephrine. Norepinephrine elevates the intracellular cAMP concentration via beta-adrenergic receptors and activates the cAMP-dependent protein kinase A (PKA). PKA phosphorylates the penultimate enzyme, the arylalkylamine N-acetyltransferase (AANAT). On exposure to (day)light, noradrenergic stimulation stops and the protein is immediately destroyed by proteasomal proteolysis.[72] Production of melatonin is again started in the evening at the point called the dim-light melatonin onset.
Blue light, principally around 460 to 480 nm, suppresses melatonin,[73] proportional to the light intensity and length of exposure. Until recent history, humans in temperate climates were exposed to few hours of (blue) daylight in the winter; their fires gave predominantly yellow light. The incandescent light bulb widely used in the 20th century produced relatively little blue light.[74] Light containing only wavelengths greater than 530 nm does not suppress melatonin in bright-light conditions.[75] Wearing glasses that block blue light in the hours before bedtime may decrease melatonin loss. Use of blue-blocking goggles the last hours before bedtime has also been advised for people who need to adjust to an earlier bedtime, as melatonin promotes sleepiness.[76]
Pharmacology
In pharmacological terms, melatonin works by activating one of two pharmacological receptors; MT1 or MT2. These are both G-protein coupled membrane receptors.[77] When used several hours before sleep according to the phase response curve for melatonin in humans, small amounts (0.3 mg[78]) of melatonin shift the circadian clock earlier, thus promoting earlier sleep onset and morning awakening.[79] In humans, 90% of orally administered exogenous melatonin is cleared in a single passage through the liver, a small amount is excreted in urine, and a small amount is found in saliva.[12]
Animals
In vertebrates, melatonin is produced in darkness, thus usually at night, by the pineal gland, a small endocrine gland[80] located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the suprachiasmatic nuclei from retinal photosensitive ganglion cells of the eyes[81][82] rather than the melatonin signal (as was once postulated). Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.
Many animals use the variation in duration of melatonin production each day as a seasonal clock.[83] In animals including humans,[84] The profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage coloring in seasonal animals.[84] In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including mynah birds[85] and hamsters.[86] Melatonin can suppress libido by inhibiting secretion of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is stimulated by melatonin.
During the night, melatonin regulates leptin, lowering its levels.
Plants
Until its identification in plants in 1987, melatonin was for decades thought to be primarily an animal neurohormone. When melatonin was identified in coffee extracts in the 1970s, it was believed to be a byproduct of the extraction process. Subsequently, however, melatonin has been found in all plants that have been investigated. It is present in all the different parts of plants, including leaves, stems, roots, fruits, and seeds in varying proportions.[87][88] Melatonin concentrations differ not only among plant species, but also between varieties of the same species depending on the agronomic growing conditions, varying from picograms to several micrograms per gram.[89][65] Notably high melatonin concentrations have been measured in popular beverages such as coffee, tea, wine, and beer, and crops including corn, rice, wheat, barley, and oats.[88] Melatonin is a poor direct antioxidant, it is, however, a highly efficient direct free radical scavenger and indirect antioxidant due to its ability to stimulate antioxidant enzymes.[90][91][92] Thus, melatonin in the human diet is believed to confer a number of beneficial health-related effects.[88][89][93] In some common foods and beverages, including coffee[88] and walnuts,[94] the concentration of melatonin has been estimated or measured to be sufficiently high to raise the blood level of melatonin above daytime baseline values.
Although a role for melatonin as a plant hormone has not been clearly established, its involvement in processes such as growth and photosynthesis is well established. Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described. Rather, melatonin performs important roles in plants as a growth regulator, as well as environmental stress protector. It is synthesized in plants when they are exposed to both biological stresses, for example, fungal infection, and nonbiological stresses such as extremes of temperature, toxins, increased soil salinity, drought, etc.[65][92][95]
Exogenous melatonin
Dietary supplement and neurohormone
Melatonin is categorized by the US Food and Drug Administration (FDA) as a dietary supplement, and is sold over-the-counter in both the US and Canada.[96] The FDA regulations applying to medications are not applicable to melatonin.[4] However, new FDA rules required that by June 2010, all production of dietary supplements must comply with "current good manufacturing practices" (cGMP) and be manufactured with "controls that result in a consistent product free of contamination, with accurate labeling."[97] The industry has also been required to report to the FDA "all serious dietary supplement related adverse events", and the FDA has (within the cGMP guidelines) begun enforcement of that requirement.[98]
As melatonin may cause harm in combination with certain medications or in the case of certain disorders, a doctor or pharmacist should be consulted before making a decision to take melatonin.[19]
In many countries, melatonin is recognized as a neurohormone and it cannot be sold over-the-counter.[99]
Food products
Melatonin has been reported in foods including cherries to about 0.17–13.46 ng/g,[100] bananas and grapes, rice and cereals, herbs, plums,[101] olive oil, wine[102] and beer. When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains.[103] When humans consume foods rich in melatonin such as banana, pineapple and orange, the blood levels of melatonin increase significantly.[104]
As reported in the New York Times in May 2011,[105] beverages and snacks containing melatonin are sold in grocery stores, convenience stores, and clubs. The FDA is considering whether these food products can continue to be sold with the label "dietary supplements". On 13 January 2010, it issued a warning letter to Innovative Beverage, creators of several beverages marketed as drinks, stating that melatonin is not approved as a food additive because it is not generally recognized as safe.[106]
History
Melatonin was first discovered in connection to the mechanism by which some amphibians and reptiles change the color of their skin.[107][108] As early as 1917, Carey Pratt McCord and Floyd P. Allen discovered that feeding extract of the pineal glands of cows lightened tadpole skin by contracting the dark epidermal melanophores.[109][110]
In 1958, dermatology professor Aaron B. Lerner and colleagues at Yale University, in the hope that a substance from the pineal might be useful in treating skin diseases, isolated the hormone from bovine pineal gland extracts and named it melatonin.[111] In the mid-70s Lynch et al. demonstrated[112] that the production of melatonin exhibits a circadian rhythm in human pineal glands.
The discovery that melatonin is an antioxidant was made in 1993.[113] The first patent for its use as a low-dose sleep aid was granted to Richard Wurtman at MIT in 1995.[114] Around the same time, the hormone got a lot of press as a possible treatment for many illnesses.[115] The New England Journal of Medicine editorialized in 2000: "With these recent careful and precise observations in blind persons, the true potential of melatonin is becoming evident, and the importance of the timing of treatment is becoming clear."[116]
Availability
Immediate-release melatonin is not tightly regulated in countries where it is available as an over-the-counter medication. It is available in doses from less than half a milligram to 5 mg or more. Immediate-release formulations cause blood levels of melatonin to reach their peak in about an hour. The hormone may be administered orally, as capsules, tablets, or liquids. It is also available for use sublingually, or as transdermal patches.
Formerly, melatonin was derived from animal pineal tissue, such as bovine. It is now synthetic and does not carry a risk of contamination or the means of transmitting infectious material.[4][117]
Prolonged release
Melatonin is available as a prolonged-release prescription drug. It releases melatonin gradually over 8–10 hours, intended to mimic the body's internal secretion profile.
In June 2007, the European Medicines Agency approved UK-based Neurim Pharmaceuticals' prolonged-release melatonin medication Circadin for marketing throughout the EU.[118] The drug is a prolonged-release melatonin, 2 mg, for patients aged 55 and older, as monotherapy for the short-term treatment (up to 13 weeks) of primary insomnia characterized by poor quality of sleep.[119][120]
Other countries' agencies that subsequently approved the drug include:
- Australian Therapeutics Goods Administration[121]
- Chile[121]
- Croatia[121]
- Icelandic Medicines Agency[122][123]
- Israeli Ministry of Health (MOH).[124]
- Norwegian Medicines Agency[123][125][126]
- South Africa[127]
- South Korean Ministry of Food and Drug Safety[127][128]
- Swiss Agency for Therapeutic Products (Swissmedic)[129]
See also
- 5-Methoxytryptamine
- 6-Hydroxymelatonin
- Agomelatine
- Health effects of sunlight exposure
- Melatonin receptor agonist#Drug design and development
- Ramelteon
- Sundowning
- Tasimelteon
References
- ↑ "Melatonin". Sleepdex. Retrieved 17 August 2011.
- ↑ Hardeland R, Pandi-Perumal SR, Cardinali DP (March 2006). "Melatonin". The International Journal of Biochemistry & Cell Biology. 38 (3): 313–6. doi:10.1016/j.biocel.2005.08.020. PMID 16219483.
- ↑ Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2012). "Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science". Journal of Experimental Botany. 63 (2): 577–97. doi:10.1093/jxb/err256. PMID 22016420.
- 1 2 3 Altun A, Ugur-Altun B (May 2007). "Melatonin: therapeutic and clinical utilization". Int. J. Clin. Pract. 61 (5): 835–45. doi:10.1111/j.1742-1241.2006.01191.x. PMID 17298593.
- ↑ Boutin JA, Audinot V, Ferry G, Delagrange P (August 2005). "Molecular tools to study melatonin pathways and actions". Trends Pharmacol. Sci. 26 (8): 412–19. doi:10.1016/j.tips.2005.06.006. PMID 15992934.
- 1 2 Hardeland R (July 2005). "Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance". Endocrine. 27 (2): 119–30. doi:10.1385/ENDO:27:2:119. PMID 16217125.
- ↑ Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (June 2001). "Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system". Ann. N. Y. Acad. Sci. 939: 200–15. doi:10.1111/j.1749-6632.2001.tb03627.x. PMID 11462772.
- 1 2 3 Brasure M, MacDonald R, Fuchs E, Olson CM, Carlyle M, Diem S, Koffel E, Khawaja IS, Ouellette J, Butler M, Kane RL, Wilt TJ (December 2015). "Management of Insomnia Disorder [Internet]". PMID 26844312.
- ↑ De Leersnyder H, Bresson JL, de Blois MC, Souberbielle JC, Mogenet A, Delhotal-Landes B, Salefranque F, Munnich A (2003). "Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome". J. Med. Genet. 40 (1): 74–78. doi:10.1136/jmg.40.1.74. PMC 1735264
 . PMID 12525548.
. PMID 12525548. - ↑ Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M (August 2000). "Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders". J. Pineal Res. 29 (1): 34–39. doi:10.1034/j.1600-079X.2000.290105.x. PMID 10949538.
- ↑ Grossman E, Laudon M, Zisapel N (2011). "Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials". Vasc Health Risk Manag. 7: 577–84. doi:10.2147/VHRM.S24603. PMC 3180511
 . PMID 21966222.
. PMID 21966222. - 1 2 3 Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassen T (November 2004). "Melatonin for treatment of sleep disorders". Evidence Report/Technology Assessment (Summary) (108): 1–7. PMID 15635761.
- ↑ Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, Hardeland R, Cardinali DP (2009). "Melatonin and melatonergic drugs on sleep: possible mechanisms of action". Int. J. Neurosci. 119 (6): 821–46. doi:10.1080/00207450802328607. PMID 19326288.
- ↑ Fornaro M, Prestia D, Colicchio S, Perugi G (September 2010). "A systematic, updated review on the antidepressant agomelatine focusing on its melatonergic modulation". Curr Neuropharmacol. 8 (3): 287–304. doi:10.2174/157015910792246227. PMC 3001221
 . PMID 21358978.
. PMID 21358978. - ↑ Turek FW, Gillette MU (November 2004). "Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists". Sleep Med. 5 (6): 523–32. doi:10.1016/j.sleep.2004.07.009. PMID 15511698.
- ↑ Wade AG, Ford I, Crawford G, McMahon AD, Nir T, Laudon M, Zisapel N (October 2007). "Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomes". Curr Med Res Opin. 23 (10): 2597–605. doi:10.1185/030079907X233098. PMID 17875243.
- ↑ Lemoine P, Zisapel N (2012). "Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia". Expert Opin Pharmacother. 13 (6): 895–905. doi:10.1517/14656566.2012.667076. PMID 22429105.
- 1 2 Liira J, Verbeek JH, Costa G, Driscoll TR, Sallinen M, Isotalo LK, Ruotsalainen JH (August 2014). "Pharmacological interventions for sleepiness and sleep disturbances caused by shift work". The Cochrane Database of Systematic Reviews. 8 (8): CD009776. doi:10.1002/14651858.CD009776.pub2. PMID 25113164.
- 1 2 Herxheimer A, Petrie KJ (2002). "Melatonin for the prevention and treatment of jet lag". Cochrane Database Syst Rev: CD001520. doi:10.1002/14651858.CD001520. PMID 12076414.
- ↑ Peres MF, Masruha MR, Zukerman E, Moreira-Filho CA, Cavalheiro EA (April 2006). "Potential therapeutic use of melatonin in migraine and other headache disorders". Expert Opinion on Investigational Drugs. 15 (4): 367–75. doi:10.1517/13543784.15.4.367. PMID 16548786.
- ↑ National Cancer Institute (May 2013). "Topics in complementary and alternative therapies (PDQ)". National Cancer Institute, National Institutes of Health. PMID 26389506. Retrieved 5 June 2013.
- ↑ Mills E, Wu P, Seely D, Guyatt G (November 2005). "Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis". J. Pineal Res. 39 (4): 360–66. doi:10.1111/j.1600-079X.2005.00258.x. PMID 16207291.
- ↑ Koppisetti S, Jenigiri B, Terron MP, Tengattini S, Tamura H, Flores LJ, Tan DX, Reiter RJ (October 2008). "Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: a review". Dig. Dis. Sci. 53 (10): 2592–603. doi:10.1007/s10620-007-0195-5. PMID 18338264.
- ↑ Meltz ML, Reiter RJ, Herman TS, Kumar KS (March 1999). "Melatonin and protection from whole-body irradiation: survival studies in mice". Mutat. Res. 425 (1): 21–27. doi:10.1016/S0027-5107(98)00246-2. PMID 10082913.
- ↑ Reiter RJ, Herman TS, Meltz ML (December 1996). "Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers". Mutat. Res. 371 (3–4): 221–8. doi:10.1016/S0165-1218(96)90110-X. PMID 9008723.
- ↑ Reiter RJ, Herman TS, Meltz ML (February 1998). "Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes". Mutat. Res. 397 (2): 203–08. doi:10.1016/S0027-5107(97)00211-X. PMID 9541644.
- 1 2 3 Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (January 2007). "One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species?". J. Pineal Res. 42 (1): 28–42. doi:10.1111/j.1600-079X.2006.00407.x. PMID 17198536.
- ↑ Shirazi A, Ghobadi G, Ghazi-Khansari M (July 2007). "A radiobiological review on melatonin: a novel radioprotector". J. Radiat. Res. 48 (4): 263–72. doi:10.1269/jrr.06070. PMID 17641465.
- ↑ Merrick L, Youssef D, Tanner M, Peiris AN (June 2014). "Does melatonin have therapeutic use in tinnitus?". Southern Medical Journal. 107 (6): 362–6. doi:10.14423/01.smj.0000450714.38550.d4. PMID 24945170.
- ↑ Braam W, Smits MG, Didden R, Korzilius H, Van Geijlswijk IM, Curfs LM (May 2009). "Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis". Dev Med Child Neurol (Meta-analysis). 51 (5): 340–49. doi:10.1111/j.1469-8749.2008.03244.x. PMID 19379289.
- 1 2 Rossignol DA, Frye RE (April 2011). "Melatonin in autism spectrum disorders: a systematic review and meta-analysis.". Dev Med Child Neurol (Meta-analysis). 53 (9): 783–92. doi:10.1111/j.1469-8749.2011.03980.x. PMID 21518346.
- ↑ Veatch OJ1, Pendergast JS, Allen MJ, Leu RM, Johnson CH, Elsea SH, Malow BA (January 2015). "Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay.". J Autism Dev Disord. 45 (1): 100–10. doi:10.1007/s10803-014-2197-4. PMID 25059483.
- ↑ "Melatonin improves sleep quality and behavior in children with asperger disorder.". Truthly. Retrieved 15 February 2015.
- ↑ Giannotti F, Cortesi F, Cerquiglini A, Bernabei P (August 2006). "An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism.". J Autism Dev Disord. 36 (6): 741–52. doi:10.1007/s10803-006-0116-z. PMID 16897403.
- ↑ Bendz LM, Scates AC (January 2010). "Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder". Ann Pharmacother. 44 (1): 185–91. doi:10.1345/aph.1M365. PMID 20028959.
- ↑ Hansen MV, Halladin NL, Rosenberg J, Gögenur I, Møller AM (2015). "Melatonin for pre- and postoperative anxiety in adults.". Cochrane Database Syst Rev (4): CD009861. doi:10.1002/14651858.CD009861.pub2.
- ↑ Lewis, Alan (1999). Melatonin and the Biological Clock. McGraw-Hill. p. 23. ISBN 0-87983-734-9.
- ↑ Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Baker G, Klassen TP, Vohra S (2005). "The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis". J Gen Intern Med. 20 (12): 1151–58. doi:10.1111/j.1525-1497.2005.0243.x. PMC 1490287
 . PMID 16423108.
. PMID 16423108. - ↑ Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G (February 2006). "Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis". BMJ. 332 (7538): 385–93. doi:10.1136/bmj.38731.532766.F6. PMC 1370968
 . PMID 16473858.
. PMID 16473858. - ↑ Lyseng-Williamson KA (2012). "Melatonin prolonged release: in the treatment of insomnia in patients aged ≥55 years". Drugs Aging. 29 (11): 911–23. doi:10.1007/s40266-012-0018-z. PMID 23044640.
- ↑ Brent Bauer, M.D. "Melatonin side effects: What are the risks?". Mayo Clinic. Retrieved 17 August 2011.
- ↑ Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU (October 2001). "Melatonin treatment for age-related insomnia". J. Clin. Endocrinol. Metab. 86 (10): 4727–30. doi:10.1210/jc.86.10.4727. PMID 11600532.
- ↑ Morera AL, Henry M, de La Varga M (2001). "Seguridad en el uso de la melatonina" [Safety in melatonin use]. Actas Esp Psiquiatr (in Spanish). 29 (5): 334–37. PMID 11602091.
- ↑ Terry PD, Villinger F, Bubenik GA, Sitaraman SV (January 2009). "Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research". Inflamm. Bowel Dis. 15 (1): 134–40. doi:10.1002/ibd.20527. PMID 18626968.
- ↑ Juszczak M, Michalska M (2006). "Wpływ melatoniny na syntezę i wydzielanie prolaktyny, hormonu luteinizującego (LH) i folikulotropowego (FSH)" [The effect of melatonin on prolactin, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) synthesis and secretion]. Postepy Hig Med Dosw (Online) (in Polish). 60: 431–38. PMID 16921343.
- ↑ Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, Brzezinski A (December 2009). "Melatonin and human reproduction: shedding light on the darkness hormone". Gynecol. Endocrinol. 25 (12): 779–85. doi:10.3109/09513590903159649. PMID 19905996.
- ↑ Cohen M, van Heusden AM, Verdonk HE, Wijnhamer P (1993). "Melatonin/Norethisterone contraception". In Touitou Y, Arendt J, Pevet P. Melatonin and the Pineal Gland – From Basic Science to Clinical Application. Amsterdam: Elsevier. pp. 339–45. ISBN 978-0-444-89583-7.
- ↑ "Possible Interactions with: Melatonin". University of Maryland Medical Center. 2007. Retrieved 27 January 2016.
Melatonin may increase the risk of bleeding from anticoagulant medications such as warfarin.
- ↑ Ardura J, Gutierrez R, Andres J, Agapito T (2003). "Emergence and evolution of the circadian rhythm of melatonin in children". Horm. Res. 59 (2): 66–72. doi:10.1159/000068571. PMID 12589109.
- ↑ Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM (1986). "Human melatonin production decreases with age". J. Pineal Res. 3 (4): 379–88. doi:10.1111/j.1600-079X.1986.tb00760.x. PMID 3783419.
- ↑ Gavin ML, Scaivina MT (2009). "Why Aren't Teens Getting Enough Sleep?". How Much Sleep Do I Need?.
- ↑ Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993). "Melatonin: a potent, endogenous hydroxyl radical scavenger". Endocrine J. 1: 57–60.
- ↑ Reiter RJ, Manchester LC, Tan DX (September 2010). "Neurotoxins: free radical mechanisms and melatonin protection". Curr Neuropharmacol. 8 (3): 194–210. doi:10.2174/157015910792246236. PMC 3001213
 . PMID 21358970.
. PMID 21358970. - ↑ Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR (November 1994). "Melatonin – a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro". Ann. N. Y. Acad. Sci. 738: 419–20. Bibcode:1994NYASA.738..419P. doi:10.1111/j.1749-6632.1994.tb21831.x. PMID 7832450.
- 1 2 Arnao MB, Hernández-Ruiz J (May 2006). "The physiological function of melatonin in plants". Plant Signal Behav. 1 (3): 89–95. doi:10.4161/psb.1.3.2640. PMC 2635004
 . PMID 19521488.
. PMID 19521488. - ↑ Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994). "Melatonin: a peroxyl radical scavenger more effective than vitamin E". Life Sci. 55 (15): PL271–76. doi:10.1016/0024-3205(94)00666-0. PMID 7934611.
- ↑ Lowes DA, Webster NR, Murphy MP, Galley HF (March 2013). "Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis". Br J Anaesth. 110 (3): 472–80. doi:10.1093/bja/aes577. PMC 3570068
 . PMID 23381720.
. PMID 23381720. - ↑ Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ (July 2005). "A review of the multiple actions of melatonin on the immune system". Endocrine. 27 (2): 189–200. doi:10.1385/ENDO:27:2:189. PMID 16217132.
- ↑ Arushanian EB, Beĭer EV (2002). "[Immunotropic properties of pineal melatonin]". Eksp Klin Farmakol (in Russian). 65 (5): 73–80. PMID 12596522.
- ↑ Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (May 2006). "The modulatory role of melatonin on immune responsiveness". Curr Opin Investig Drugs. 7 (5): 423–31. PMID 16729718.
- ↑ Maestroni GJ (March 2001). "The immunotherapeutic potential of melatonin". Expert Opin Investig Drugs. 10 (3): 467–76. doi:10.1517/13543784.10.3.467. PMID 11227046.
- ↑ Limson J, Nyokong T, Daya S (1998). "The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study". Journal of Pineal Research. 24 (1): 15–21. doi:10.1111/j.1600-079x.1998.tb00361.x. PMID 9468114.
- ↑ "MetaCyc serotonin and melatonin biosynthesis".
- ↑ Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (January 2012). "Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources". Journal of Chemical Biology. 5 (1): 5–17. doi:10.1007/s12154-011-0064-8. PMC 3251648
 . PMID 22826715.
. PMID 22826715. - 1 2 3 Hardeland R (February 2015). "Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions". Journal of Experimental Botany. 66 (3): 627–46. doi:10.1093/jxb/eru386. PMID 25240067.
- ↑ Roberts KM, Fitzpatrick PF (2013). "Mechanisms of tryptophan and tyrosine hydroxylase". IUBMB Life. 65 (4): 350–57. doi:10.1002/iub.1144. PMC 4270200
 . PMID 23441081.
. PMID 23441081. - ↑ Sumi-Ichinose C, Ichinose H, Takahashi E, Hori T, Nagatsu T (1992). "Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis". Biochemistry. 31 (8): 2229–38. doi:10.1021/bi00123a004. PMID 1540578.
- ↑ Dewick PM (2002). Medicinal Natural Products. A Biosynthetic Approach (2nd ed.). Wiley. ISBN 0-471-49640-5.
- ↑ Hickman AB, Klein DC, Dyda F (1999). "Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism". Mol. Cell. 3 (1): 23–32. doi:10.1016/S1097-2765(00)80171-9. PMID 10024876.
- ↑ Dewick PM (2002). Medicinal Natural Products. A Biosynthetic Approach (2nd ed.). Wiley. ISBN 0-471-49640-5.
- ↑ Donohue SJ, Roseboom PH, Illnerova H, Weller JL, Klein DC (1993). "Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA". DNA Cell Biol. 12 (8): 715–27. doi:10.1089/dna.1993.12.715. PMID 8397829.
- ↑ Schomerus C, Korf HW (December 2005). "Mechanisms regulating melatonin synthesis in the mammalian pineal organ". Annals of the New York Academy of Sciences. 1057 (1): 372–83. doi:10.1196/annals.1356.028. PMID 16399907.
- ↑ Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD (August 2001). "Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor". J. Neurosci. 21 (16): 6405–12. PMID 11487664.
- ↑ Cornell University, Light source spectra
- ↑ Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM (May 2005). "Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work". J. Clin. Endocrinol. Metab. 90 (5): 2755–61. doi:10.1210/jc.2004-2062. PMID 15713707.
- ↑ Burkhart K, Phelps JR (26 December 2009). "Amber lenses to block blue light and improve sleep: a randomized trial". Chronobiol Int. 26 (8): 1602–12. doi:10.3109/07420520903523719. PMID 20030543.
- ↑ http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39
- ↑ Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC (October 2005). "Phase-dependent treatment of delayed sleep phase syndrome with melatonin". Sleep. 28 (10): 1271–78. PMID 16295212.
- ↑ Terman MR, Wirz-Justice A (2009). Chronotherapeutics for Affective Disorders: A Clinician's Manual for Light and Wake Therapy. Basel: S Karger Pub. p. 71. ISBN 3-8055-9120-9.
- ↑ Reiter RJ (May 1991). "Pineal melatonin: cell biology of its synthesis and of its physiological interactions". Endocr. Rev. 12 (2): 151–80. doi:10.1210/edrv-12-2-151. PMID 1649044.
- ↑ Richardson GS (2005). "The human circadian system in normal and disordered sleep". J Clin Psychiatry. 66 Suppl 9: 3–9; quiz 42–3. PMID 16336035.
- ↑ Perreau-Lenz S, Pévet P, Buijs RM, Kalsbeek A (January 2004). "The biological clock: the bodyguard of temporal homeostasis". Chronobiol. Int. 21 (1): 1–25. doi:10.1081/CBI-120027984. PMID 15129821.
- ↑ Lincoln GA, Andersson H, Loudon A (October 2003). "Clock genes in calendar cells as the basis of annual timekeeping in mammals – a unifying hypothesis". J. Endocrinol. 179 (1): 1–13. doi:10.1677/joe.0.1790001. PMID 14529560.
- 1 2 Arendt J, Skene DJ (February 2005). "Melatonin as a chronobiotic". Sleep Med Rev. 9 (1): 25–39. doi:10.1016/j.smrv.2004.05.002. PMID 15649736.
Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn), it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times.
- ↑ Chaturvedi CM (1984). "Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis". Australian Journal of Zoology. 32 (6): 803–09. doi:10.1071/ZO9840803.
- ↑ Chen HJ (July 1981). "Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition". Neuroendocrinology. 33 (1): 43–46. doi:10.1159/000123198. PMID 7254478.
- ↑ Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009-01-01). "Phytomelatonin: a review". Journal of Experimental Botany. 60 (1): 57–69. doi:10.1093/jxb/ern284. PMID 19033551.
- 1 2 3 4 Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (January 2012). "Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science". Journal of Experimental Botany. 63 (2): 577–97. doi:10.1093/jxb/err256. PMID 22016420.
- 1 2 Bonnefont-Rousselot D, Collin F (November 2010). "Melatonin: action as antioxidant and potential applications in human disease and aging". Toxicology. 278 (1): 55–67. doi:10.1016/j.tox.2010.04.008. PMID 20417677.
- ↑ Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996). "Evaluation of the antioxidant activity of melatonin in vitro". Free Radical Biology & Medicine. 21 (3): 307–15. doi:10.1016/0891-5849(96)00046-9. PMID 8855441.
- ↑ Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (February 2002). "Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger". Current Topics in Medicinal Chemistry. 2 (2): 181–97. doi:10.2174/1568026023394443. PMID 11899100.
- 1 2 Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A (April 2015). "Phytomelatonin: assisting plants to survive and thrive". Molecules. 20 (4): 7396–437. doi:10.3390/molecules20047396. PMID 25911967.
- ↑ Iriti M, Varoni EM, Vitalini S (September 2010). "Melatonin in traditional Mediterranean diets". Journal of Pineal Research. 49 (2): 101–5. doi:10.1111/j.1600-079X.2010.00777.x. PMID 20536683.
- ↑ Reiter RJ, Manchester LC, Tan DX (September 2005). "Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood". Nutrition. 21 (9): 920–4. doi:10.1016/j.nut.2005.02.005. PMID 15979282.
- ↑ Arnao MB, Hernández-Ruiz J (September 2015). "Functions of melatonin in plants: a review". Journal of Pineal Research. 59 (2): 133–50. doi:10.1111/jpi.12253. PMID 26094813.
- ↑ Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassen T (November 2004). "Melatonin for treatment of sleep disorders" (PDF). Evidence Report/Technology Assessment No. 108. (Prepared by the University of Alberta Evidence-based Practice Center, under Contract No. 290-02-0023.) AHRQ Publication No. 05-E002-2. Rockville, MD: Agency for Healthcare Research and Quality. Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services. Retrieved 5 June 2013.
- ↑ "FDA Issues Dietary Supplements Final Rule" (Press release). U.S. Food and Drug Administration. 22 June 2007. Retrieved 4 August 2009.
- ↑ "FDA Tightens Up Dietary Supplement Manufacturing And Labelling". Medical News Today. 26 June 2007. Retrieved 2 September 2013.
- ↑ Guardiola-Lemaître B (December 1997). "Toxicology of melatonin". Journal of Biological Rhythms. 12 (6): 697–706. doi:10.1177/074873049701200627. PMID 9406047.
- ↑ Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (October 2001). "Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus)". J. Agric. Food Chem. 49 (10): 4898–902. doi:10.1021/jf010321. PMID 11600041.
- ↑ González-Flores D, Velardo B, Garrido M, González-Gómez D, Lozano M, Ayuso MC, Barriga C, Paredes SD, Rodríguez AB (2011). "Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content". Journal of Food and Nutrition Research. 50 (4): 229–36.
- ↑ Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (May 2011). "Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection". J. Pi JJ Res. 50 (4): 374–80. doi:10.1111/j.1600-079X.2010.00853.x. PMID 21342247.
- ↑ Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (March 1995). "Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates". Biochem. Mol. Biol. Int. 35 (3): 627–34. PMID 7773197.
- ↑ Sae-Teaw M, Johns J, Johns NP, Subongkot S (October 2012). "Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers". J. Pineal Res. 55 (1): 58–64. doi:10.1111/jpi.12025. PMID 23137025.
- ↑ Catherine Saint Louis (14 May 2011). "Dessert, Laid-Back and Legal". New York Times.
- ↑ Rodriguez RR (13 January 2010). "Warning Letter". Inspections, Compliance, Enforcement, and Criminal Investigations. U.S. Food and Drug Administration.
- ↑ Filadelfi AM, Castrucci AM (May 1996). "Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates". J. Pineal Res. 20 (4): 175–86. doi:10.1111/j.1600-079X.1996.tb00256.x. PMID 8836950.
- ↑ Sugden D, Davidson K, Hough KA, Teh MT (October 2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Res. 17 (5): 454–60. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- ↑ Coates PM, Blackman MR, Cragg GM, LevineM, Moss J, White JD (2005). Encyclopedia of dietary supplements. New York, N.Y: Marcel Dekker. pp. 457–66. ISBN 0-8247-5504-9.
- ↑ McCord CP, Allen FP (January 1917). "Evidences associating pineal gland function with alterations in pigmentation". J Exptl Zool. 23 (1): 206–24. doi:10.1002/jez.1400230108.
- ↑ Lerner AB, Case JD, Takahashi Y (July 1960). "Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands". J. Biol. Chem. 235: 1992–97. PMID 14415935.
- ↑ Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH (January 1975). "Daily rhythm in human urinary melatonin". Science. 187 (4172): 169–71. Bibcode:1975Sci...187..169L. doi:10.1126/science.1167425. PMID 1167425.
- ↑ Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC (May 1993). "Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis". J. Pineal Res. 14 (4): 151–68. doi:10.1111/j.1600-079X.1993.tb00498.x. PMID 8102180.
- ↑ US patent 5449683, Wurtman RJ, "Methods of inducing sleep using melatonin", issued 12 September 1995, assigned to Massachusetts Institute of Technology
- ↑ Arendt J (August 2005). "Melatonin: characteristics, concerns, and prospects". J. Biol. Rhythms. 20 (4): 291–303. doi:10.1177/0748730405277492. PMID 16077149.
There is very little evidence in the short term for toxicity or undesirable effects in humans. The extensive promotion of the miraculous powers of melatonin in the recent past did a disservice to acceptance of its genuine benefits.
- ↑ Arendt J (October 2000). "Melatonin, circadian rhythms, and sleep". N. Engl. J. Med. 343 (15): 1114–16. doi:10.1056/NEJM200010123431510. PMID 11027748.
- ↑ "Melatonin". Drugs.com. Retrieved 17 August 2011.
- ↑ European Medicines Agency (29 June 2007). "Circadin: Authorisation details". European Medicines Agency. Retrieved 5 August 2015.
- ↑ Medical News Today Circadin (Prolonged-Release Melatonin) For Primary Insomnia Recommended For Approval In The EU (27 April 2007)
- ↑ European Medicines Agency. "Circadin, melatonin". European Public Assessment Report (EPAR). European Medicines Agency. Retrieved 5 June 2013.
- 1 2 3 Therapeutic Goods Administration (TGA). "Australian Public Assessment Report for Melatonin" (PDF). Department of Health and Ageing, Australian Government. Retrieved 5 June 2013.
- ↑ "Lyf með markaðsleyfi á Íslandi" (PDF). Icelandic Medicines Agency (in Icelandic). 1 August 2015. p. 65. Retrieved 20 August 2015.
Circadin ... Lyfseðilsskylt (prescription) ... Yes (marketed) ... 8 August 2007(marketing authorization issued)
- 1 2 Lundbeck (13 May 2008). "Lundbeck Release: Novel Treatment Offers Insomnia Sufferers Hope of Quality Sleep". BioSpace. BioSpace.com. Retrieved 20 August 2015.
At the end of February 2008, Circadin(R) had been launched by Nycomed in ... Iceland, ... and Norway.
- ↑ Ministry of Health Israel. "Circadin leaflet".
- ↑ Bjorvatn, Bjørn. "Behandling av søvnproblemer med melatonin". Nasjonal kompetansetjeneste for søvnsykdommer (SOVno) (in Norwegian). Helse-Bergen. Retrieved 8 April 2015.
I 2008 ble Circadin (...) tilgjengelig på vanlig hvit resept i Norge.
- ↑ "Melatoninpreparater og godkjenningsfritak". Statens Legemiddelverk (in Norwegian). Statens Legemiddelverk. Retrieved 8 April 2015.
- 1 2 "Melatonin controlled-release - Neurim Pharmaceuticals". Adis Insight. Springer. 7 Apr 2015. Retrieved 30 Aug 2015.
07 Sep 2014 Registered for Insomnia in South Africa, 01 Sep 2014 Registered for Insomnia in South Korea
- ↑ "Circadin license application" (PDF) (in Korean). MFDS. 2014.
- ↑ SwissMedic (2009). "Circadin®, Retardtabletten, 2 mg (melatoninum)" (in German).
Further reading
- Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N (2010). "Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety". BMC Med. 8: 51. doi:10.1186/1741-7015-8-51. PMC 2933606
 . PMID 20712869.
. PMID 20712869.
External links
| Wikimedia Commons has media related to Melatonin. |