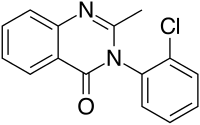
Mecloqualone
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
340-57-8 |
| PubChem (CID) | 9567 |
| ChemSpider |
9192 |
| UNII |
09XU4VDV7E |
| KEGG |
D04877 |
| ChEMBL |
CHEMBL279960 |
| ECHA InfoCard | 100.005.848 |
| Chemical and physical data | |
| Formula | C15H11ClN2O |
| Molar mass | 270.714 |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Mecloqualone (Nubarene, Casfen) is a quinazolinone-class GABAergic and is an analogue of methaqualone that was first made in 1960[1] and marketed mainly in France and some other European countries. It has sedative, hypnotic, and anxiolytic properties caused by its agonist activity at the β subtype of the GABAa receptor, and was used for the treatment of insomnia.[2] Mecloqualone is faster-acting but shorter-lasting than methaqualone and so was used only as a sleeping pill,[3] in contrast to methaqualone, which was used as a general-purpose anxiolytic as well. Mecloqualone was never as widely used as methaqualone and is no longer prescribed because of concerns about its potential for abuse and overdose. In the United States it is a Schedule I non-narcotic (depressant) controlled substance with an ACSCN of 2572 and zero annual aggregate manufacturing quota.
See also
- Methaqualone
- Afloqualone
- Etaqualone
- Methylmethaqualone
- Mebroqualone
- Cloroqualone
- Diproqualone
- Gamma-Aminobutyric acid
References
- ↑ Jackman, G. B.; Petrow, V.; Stephenson, O. (1960). "Some 2, 3-disubstituted 3H-4-quinazolones and 3H-4-thioquinazolones". The Journal of pharmacy and pharmacology. 12: 529–538. PMID 14406263.
- ↑ Mouren, P.; Giraud, F.; Pinsard, N. (1963). "Clinical use of a new psycholeptic: Mecloqualone". Marseille medical. 100: 599–602. PMID 13936358.
- ↑ Dubnk, B.; Towne, C. A.; Bush, M. T. (1969). "Detection, assay and rate of excretion of mecloqualone in animals and man". Toxicology and applied pharmacology. 15 (3): 632–641. doi:10.1016/0041-008X(69)90065-9. PMID 5353825.