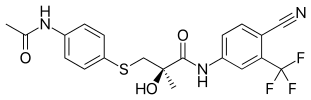
Acetothiolutamide
 | |
| Clinical data | |
|---|---|
| Routes of administration | Subcutaneous, intravenous |
| Identifiers | |
| |
| CAS Number | 216665-38-2 |
| PubChem (CID) | 10873814 |
| ChemSpider | 9049091 |
| ChEMBL | CHEMBL121940 |
| Chemical and physical data | |
| Formula | C20H18F3N3O3S |
| Molar mass | 437.43543 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Acetothiolutamide is a selective androgen receptor modulator (SARM) derived from the non-steroidal antiandrogen bicalutamide that was described in 2002 and was one of the first SARMs to be discovered and developed.[1][2][3][4] It is a high-affinity, selective ligand of the androgen receptor (AR) (Ki = 2.1–4.9 nM), where it acts as a full agonist in vitro, and has in vitro potency comparable to that of testosterone.[2][4][5] However, in vivo, acetothiolutamide displayed overall negligible androgenic effects, though significant (albeit very low) anabolic effects were observed at high doses.[2] In addition, notable antiandrogen effects were observed in castrated male rats treated with testosterone propionate.[2] The discrepancy between the in vitro and in vivo actions of acetothiolutamide was determined to be related to rapid plasma clearance and extensive hepatic metabolism into a variety of metabolites with differing pharmacological activity, including AR partial agonism and antagonism.[2][4][6] In accordance with its poor metabolic stability, acetothiolutamide is not orally bioavailable, and shows activity only via injected routes such as subcutaneous and intravenous.[2]
See also
References
- ↑ Dalton, James T.; Mukherjee, Arnab; Zhu, Zixin; Kirkovsky, Leonid; Miller, Duane D. (1998). "Discovery of Nonsteroidal Androgens". Biochemical and Biophysical Research Communications. 244 (1): 1–4. doi:10.1006/bbrc.1998.8209. ISSN 0006-291X.
- 1 2 3 4 5 6 Yin, D. (2002). "Pharmacology, Pharmacokinetics, and Metabolism of Acetothiolutamide, a Novel Nonsteroidal Agonist for the Androgen Receptor". Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1323–1333. doi:10.1124/jpet.102.040832. ISSN 0022-3565.
- ↑ Jeffrey Dale Kearbey (2004), Preclinical Pharmacokinetics and Skeletal Pharmacology of a Selective Androgen Receptor Modulator
- 1 2 3 Perera, M. A. (2006). "In Vivo Metabolism and Final Disposition of a Novel Nonsteroidal Androgen in Rats and Dogs". Drug Metabolism and Disposition. 34 (10): 1713–1721. doi:10.1124/dmd.106.009985. ISSN 0090-9556.
- ↑ Kim, J. (2005). "The Para Substituent of S-3-(Phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamides Is a Major Structural Determinant of in Vivo Disposition and Activity of Selective Androgen Receptor Modulators". Journal of Pharmacology and Experimental Therapeutics. 315 (1): 230–239. doi:10.1124/jpet.105.088344. ISSN 0022-3565.
- ↑ Yin, D. (2002). "Pharmacodynamics of Selective Androgen Receptor Modulators". Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1334–1340. doi:10.1124/jpet.102.040840. ISSN 0022-3565.