BOMT
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | None |
| Identifiers | |
| |
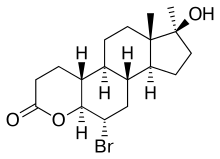
| Synonyms | Ro 7-2340; 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone |
| CAS Number |
24543-59-7 24543-66-6 |
| PubChem (CID) | 159972 |
| ChemSpider | 140636 |
| Chemical and physical data | |
| Formula | C19H29BrO3 |
| Molar mass | 385.33576 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
BOMT (developmental code name Ro 7-2340), also known as 6α-bromo-4-oxa-17α-methyl-5α-androstan-17β-ol-3-one, is a synthetic, steroidal, pure antiandrogen that was first developed in 1970 and was never marketed for clinical use.[1][2][3][4] It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of dihydrotestosterone (DHT). Along with benorterone, cyproterone (and its acetate ester, cyproterone acetate), and flutamide,[5] BOMT was among the earliest antiandrogens to be developed and extensively studied,[2][3][6][7][8] although it is less well-documented in comparison to the others.[9] BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue.[10] There was also interest in BOMT for the potential applications of acne, androgenetic alopecia, and possibly prostate cancer, but it was not developed for these indications either.[11]
BOMT is a selective competitive antagonist of the androgen receptor (AR),[3][4][12][13][14] although it is described as an "only relatively weak competitor."[15] The drug shows no androgenic, estrogenic, or progestogenic activity even at high doses, nor any inhibition of 5α-reductase,[16] though it does show weak antigonadotropic effects.[3][4][12][13] Due to its selectivity for and competitive inhibition of the AR, BOMT has been described as a "true" antiandrogen, similarly to benorterone, cyproterone, and flutamide.[17] Like other steroidal antiandrogens, BOMT may actually be a weak partial agonist of the AR, as it appears to have the potential for weak androgenic effects in specific situations.[18] On the basis of animal research, BOMT does not appear to act as an AR antagonist in central nervous system tissues, and in relation to this, does not disinhibit the hypothalamic-pituitary-gonadal axis or increase testosterone levels.[19]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 178–. ISBN 978-1-4757-2085-3.
- 1 2 Boris, A.; Uskokovic, M. (1970). "A new antiandrogen. 6α-Bromo-17β-hydroxy-17α-methyl-4-oxa-5α-androstan-3-one". Experientia. 26 (1): 9–10. doi:10.1007/BF01900355. ISSN 0014-4754.
[It is shown that 6α-bromo-17β-hydroxy-17α-methyl-4-oxa-5α-androstan-3-one, has significant anti-androgenic activity. Isomers of this compound with different configuration at C-5 and C-6 were found to be inactive.]
- 1 2 3 4 Boris, Alfred; Demartino, Louis; Trmal, Thelma (1971). "Some Endocrine Studies of a New Antiandrogen, 6 α-Bromo-17β-hydroxy-17α-methyl-4-oxa-5α:-androstan-3-one (BOMT)". Endocrinology. 88 (4): 1086–1091. doi:10.1210/endo-88-4-1086. ISSN 0013-7227.
- 1 2 3 Mangan, F.R.; Mainwaring, W.I.P. (1972). "An explanatiom of the antiandrogenic properties of 6α-bromo-17β-hydroxy-17α-methyl-4-oxa-5α-androstane-3-one". Steroids. 20 (3): 331–343. doi:10.1016/0039-128X(72)90092-X. ISSN 0039-128X.
- ↑ T. Mann; C. Lutwak-Mann (6 December 2012). Male Reproductive Function and Semen: Themes and Trends in Physiology, Biochemistry and Investigative Andrology. Springer Science & Business Media. pp. 352–. ISBN 978-1-4471-1300-3.
- ↑ W.I.P. Mainwaring (6 December 2012). The Mechanism of Action of Androgens. Springer Science & Business Media. pp. 10–. ISBN 978-3-642-88429-0.
- ↑ Bentham Science Publishers (December 1999). Current Medicinal Chemistry. Bentham Science Publishers. pp. 1110–1111.
Several androstane derivatives have also demonstrated an antiandrogenic activity; 17α-methyl-B-nortestosterone 8 was prepared and tested in 1964 for antihormonal activity [43]. Within the next decades, several other androstane analogs were prepared and found to possess antiandrogenic activity [43, 44, 45, 46] including BOMT 9 "figure 2", R2956 10, SC9420 11, and oxendolone 12 "figure 3".
- ↑ Anthony W. Norman; Gerald Litwack (28 June 2014). Hormones. Elsevier Science. pp. 508–. ISBN 978-1-4832-5810-2.
- ↑ Clark, C.R.; Nowell, N.W. (1979). "Binding properties of testosterone receptors in the hypothalamic-preoptic area of the adult kale mouse brain". Steroids. 33 (4): 407–426. doi:10.1016/0039-128X(79)90015-1. ISSN 0039-128X.
However, a less well documented antiandrogen, BOMT possesses the ideal characteristics of negligible androgenic, estrogenic and progestational activity (55) and would therefore appear to be a valuable compound for use in future investigations.
- ↑ John Kent; Arthur Bischoff; Harry Herr & William O'Connell (1973), Study of antiandrogen (Ro–7–2340) (6α-bromo-17β-methyl-4-oxa-5α-andronstan-3-one) in benign prostatic hypertrophy
- ↑ Bert O'Malley (11 November 2013). Receptors for Reproductive Hormones. Springer Science & Business Media. pp. 208–. ISBN 978-1-4684-3237-4.
- 1 2 Brian Peter Setchell (1978). The mammalian testis. P. Elek. p. 144. ISBN 978-0-236-31057-9.
Another steroidal compound with anti-androgenic activity is BOMT (6α-bromo-17β-hydroxy-17α-methyl-4-oxa-5α-androstan-3-one). This compound has no androgenic, oestrogenic or progestational activity but is a potent anti-androgen (Boris et al., 1970); it competes effectively for the specific, high-affinity binding sites for DHT in the rat prostate (Mangan and Mainwaring, 1972) and depresses testis weight (Boris et al., 1970).
- 1 2 R. J. B. King; W. I. P. Mainwaring (20 May 2014). Steroid–Cell Interactions. Elsevier. pp. 52,61,70–71,300,401–403. ISBN 978-1-4831-6510-3.
- ↑ Singh, Shankar; Gauthier, Sylvain; Labrie, Fernand (2000). "Androgen Receptor Antagonists (Antiandrogens) Structure-Activity Relationships" (PDF). Current Medicinal Chemistry. 7 (2): 211–247. doi:10.2174/0929867003375371. ISSN 0929-8673.
- ↑ Heyns, W.; G., Verhoeven; De Moor, P. (1976). "Androgen binding in rat uterus cytosol. Study of the specificity". Journal of Steroid Biochemistry. 7 (5): 335–343. doi:10.1016/0022-4731(76)90092-3. ISSN 0022-4731.
Finally, the steroidal antiandrogen BOMT and the non-steroidal antiandrogen DIMP are only relatively weak competitors.
- ↑ Mangan, F.R.; Mainwaring, W.I.P. (1971). "The Biochemical Basis for the Antagonism hy BOMT of the Effects of Dihydrotesterone on the Rat Ventral Prostate Gland". Gynecologic and Obstetric Investigation. 2 (1-6): 300–304. doi:10.1159/000301871. ISSN 1423-002X.
- ↑ Tremblay, Roland R. (1986). "10 Treatment of hirsutism with spironolactone". Clinics in Endocrinology and Metabolism. 15 (2): 363–371. doi:10.1016/S0300-595X(86)80030-5. ISSN 0300-595X.
Flutamide, cyproterone, benorterone, RU-2956, BOMT and cimetidine are recognized as true antiandrogens because they act as competitive inhibitors of specific ligand binding to androgen receptors.
- ↑ Ahlin K, Forsberg JG, Jacobsohn D, Thore-Berger B (1975). "The male genital tract and the nipples of male and female offspring of rats given the non-steroidal antiandrogens DIMP and Sch 13521, during pregnancy". Arch Anat Microsc Morphol Exp. 64 (1): 27–44. PMID 1217898.
- ↑ Clark, C.R.; Nowell, N.W. (1979). "Bomt (6α-bromo-17β-hydroxy-17α-methyl-4-oxa-5α-androstan-3-one) is not an androgen antagonist within the central nervous system". Steroids. 34 (2): 139–149. doi:10.1016/0039-128X(79)90043-6. ISSN 0039-128X.