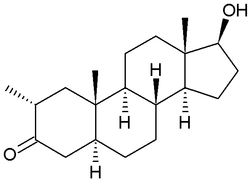
Drostanolone
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
58-19-5 |
| PubChem (CID) | 6011 |
| ChemSpider |
5789 |
| UNII |
7DR7H00HDT |
| ChEMBL |
CHEMBL1582 |
| Chemical and physical data | |
| Formula | C20H32O2 |
| Molar mass | 304.46 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Drostanolone (INN), also known as dromostanolone,[1] is an anabolic-androgenic steroid (AAS).[2][3][4] Drostanolone is a part of the dihydrotestosterone (DHT) family. Drostanolone itself has never been marketed; it is instead used as the ester prodrug drostanolone propionate (brand names Masteron, Drolban).[2][3] Its main medical uses (as drostanolone propionate) include lowering cholesterol levels and as an antineoplastic agent [5] in the treatment of some cancers.[6]
Drostanolone binds to the androgen receptor and activates a cascade of genetic changes, which increases the protein synthesis (anabolism) and decreases the amino acids degradation (catabolism). Other effects of drostanolone are reduction or inhibition of prolactin or estrogen receptors, which are linked to its antitumor properties.[7]
Usage
Breast cancer
Hormonal treatment is part of the complex therapy for some kind of tumors, particularly the ones associated with hormone-active tissues like breast or prostate cancer. Some types of breast cancer cells, expressing estrogen receptors (called ER+ cancers), use estrogen for their growth and dissemination. That is why drugs that block estrogen receptors or decrease their expression on the cell membrane- anti-estrogens- could limit the tumor spread and size. Drostanolone has been FDA approved[8] as an anti-estrogenic drug for the treatment of breast cancer. By the time of its release, there were not many alternatives for patients suffering from breast cancer and drostanolone was a revolution for these patients. As it has lower androgenic rate compared to testosterone, the risk of virilization is much lighter. Due to this fact, women, who usually do not respond well to any anabolic steroids, were having much greater chance to survive cancer. Drostanolone can also be used for breast tumors that do not respond well to other treatments or also as palliative care for advanced incurable tumors. The effects of the product depend of course on the dose and period of administration. The risk of virilization becomes greater with high doses and continuous administration period.
Bodybuilding
With the development of more precise treatment approaches, drostanolone as an antitumor agent is being replaced by medicaments with lower virilization risk and reduced side effects. However, drostanolone remains popular amongst bodybuilders, especially during cutting cycles, when fat loss is the main target. It should be emphasized that drostanolone will be only effective if the user's body fat percentage is already low (<10%). If this is the case, drostanolone will not only help with the last stubborn bit of fat, but will also improve the physique, making it appear harder and leaner by underlining muscle separation. This makes drostanolone popular for pre-contest cycles. These effects of drostanolone are due to its inability to aromatize into estrogen.[9]
Many bodybuilders combine drostanolone with other anabolic steroids. Drostanolone itself is only weakly anabolic, which is why it is not used alone during bulking cycles. However, when combined with other anabolic steroids, drostanolone may enhance their effects. Because drostanolone acts as an anti-estrogen through the suppression of estrogen receptors, other aromatizable steroids have less pronounced estrogen-related side effects. Also, drostanolone binds sex hormone-binding globulin (SHBG) which usually will bind a portion of free sex hormones and steroids, like testosterone, and inactivate them. When drostanolone binds to SHBG, the level of unbound SHBG drops, lowering the chance of it binding to other steroids, which increases their bioavailablilty.
In addition, drostanolone is also suitable for those following calories restricted regime as it increases strength, reduces recovery periods and improves endurance without weight gain.
Side effects
As drostanolone does not aromatize at any dose, typical estrogen-linked side effects encountered when using other anabolic steroids, like water retention, gynecomastia, and elevated blood pressure are not observed.
However, androgenic effects such as accelerated alopecia and acne can appear. In women, drostanolone can cause virilization when used in high doses. Drostanolone can also cause a decrease in HDL cholesterol and an increase in LDL cholesterol. As drostanolone also suppresses natural testosterone production, additional testosterone therapy and post-cycle therapy should be considered.[10]
References
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 2016-03-15.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- ↑ "SAFS | forendex | Drostanolone propionate". forendex.southernforensic.org. Retrieved 2016-03-15.
- ↑ Gauthier, Julie; Goudreault, Danielle; Poirier, Donald; Ayotte, Christiane (2009-03-01). "Identification of drostanolone and 17-methyldrostanolone metabolites produced by cryopreserved human hepatocytes". Steroids. 74 (3): 306–314. doi:10.1016/j.steroids.2008.11.002. ISSN 0039-128X. PMID 19056412.
- ↑ Bennett, MB; Helman, P; Palmer, P (1975). "Hormonal therapy of breast cancer with special reference to Masteril therapy". S. Afr. Med. J. 49: 2036–40. PMID 1242823.
- ↑ "Drostanolone (PIM 901)". www.inchem.org. Retrieved 2016-03-15.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 2016-03-15.
- ↑ "DB00858 - 'Drostanolone'". www.ebi.ac.uk. Retrieved 2016-03-15.
- ↑ "Drostanolone (PIM 901)". www.inchem.org. Retrieved 2016-03-15.
Further reading
- Ringold, H. J.; Batres, E.; Halpern, O.; Necoechea, E.; J. Amer. Chem. Soc. 1959, 81, 427.