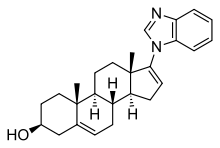
Galeterone
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | 851983-85-2 |
| PubChem (CID) | 11188409 |
| ChemSpider | 9363493 |
| UNII |
WA33E149SW |
| KEGG |
D10125 |
| Chemical and physical data | |
| Formula | C26H32N2O |
| Molar mass | 388.25 |
| 3D model (Jmol) | Interactive image |
| |
| |
Galeterone (TOK-001 or VN/124-1) is a novel steroidal antiandrogen under development by Tokai Pharmaceuticals for the treatment of prostate cancer. It possesses a unique dual mechanism of action, acting as both an androgen receptor antagonist and an inhibitor of CYP17A1, an enzyme required for the biosynthesis of the androgens.[1] It shows selectivity for 17,20-lyase over 17-hydroxylase.[2]
Galeterone was being compared to enzalutamide in a phase III clinical trial (ARMOR3-SV) for AR-V7-expressing metastatic castration-resistant prostate cancer.[3][4] Tokai announced the discontinuation of ARMOR3-SV on July 26, 2016, after a data monitoring committee determined that the trial was unlikely to meet its endpoint.[5] On August 22, 2016, the company announced the discontinuation of their phase II expansion (ARMOR2) as well.[6]
See also
References
- ↑ Brawer MK (2008). "New treatments for castration-resistant prostate cancer: highlights from the 44th annual meeting of the american society of clinical oncology, may 30-june 3, 2008, chicago, IL". Rev Urol. 10 (4): 294–6. PMC 2615106
 . PMID 19145273.
. PMID 19145273. - ↑ Yin L, Hu Q (2014). "CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents". Nat Rev Urol. 11 (1): 32–42. doi:10.1038/nrurol.2013.274. PMID 24276076.
- ↑ "A Study of Galeterone Compared to Enzalutamide In Men Expressing Androgen Receptor Splice Variant-7 mRNA (AR-V7) Metastatic CRPC - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-02-27.
- ↑ Silberstein, John L.; Taylor, Maritza N.; Antonarakis, Emmanuel S. (2016-04-01). "Novel Insights into Molecular Indicators of Response and Resistance to Modern Androgen-Axis Therapies in Prostate Cancer". Current Urology Reports. 17 (4): 29. doi:10.1007/s11934-016-0584-4. ISSN 1534-6285. PMC 4888068
 . PMID 26902623.
. PMID 26902623. - ↑ http://www.businesswire.com/news/home/20160726005553/en/Tokai-Pharmaceuticals-Announces-Clinical-Update
- ↑ http://seekingalpha.com/news/3204773-tokai-pharma-flux-lead-product-candidate-galeterone-enrollment-mid-stage-prostate-cancer