Macimorelin
 | |
| Names | |
|---|---|
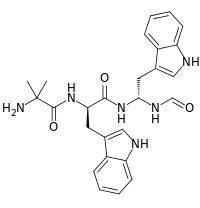
| IUPAC name
2-Amino-N-[(2R)-1-[[(1R)-1-formamido-2-(1H-indol-3-yl)ethyl]amino]-3-1H-indol-3-yl)-1-oxopropan-2-yl]-2-methylpropanamide | |
| Other names
Aib-Trp-gTrp-CHO; AEZS-130; JMV 1843; Macimorelin acetate | |
| Identifiers | |
| 381231-18-1 945212-59-9 (acetate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7980698 |
| PubChem | 9804938 |
| |
| |
| Properties | |
| C26H30N6O3 | |
| Molar mass | 474.57 g·mol−1 |
| Pharmacology | |
| V04CD06 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Macimorelin (INN) is a drug being developed by Æterna Zentaris for use in the diagnosis of adult growth hormone deficiency. As of January 2014, it is in Phase III clinical trials.[1]
Macimorelin is a mimic of ghrelin, a growth hormone secretagogue. It binds to the growth hormone secretagogue receptor (GHSR) causing release of growth hormone from the pituitary gland.[2][3][4]
See also
- Anamorelin
- Capromorelin
- Examorelin (hexarelin)
- GHRP-6 (SKF-110679)
- Ibutamoren (MK-677)
- Ipamorelin
- Pralmorelin (GHRP-2)
- Relamorelin
- SM-130,686
- Tabimorelin
References
- ↑ "Aeterna Zentaris NDA for Macimorelin Acetate in AGHD Accepted for Filing by the FDA". Wall Street Journal. January 6, 2014.
- ↑ "Macimorelin". NCI Drug Dictionary. National Cancer Institute.
- ↑ Koch, Linda (2013). "Growth hormone in health and disease: Novel ghrelin mimetic is safe and effective as a GH stimulation test". Nature Reviews Endocrinology. 9 (6): 315. doi:10.1038/nrendo.2013.89.
- ↑ Garcia, J. M.; Swerdloff, R.; Wang, C.; Kyle, M.; Kipnes, M.; Biller, B. M. K.; Cook, D.; Yuen, K. C. J.; Bonert, V.; Dobs, A.; Molitch, M. E.; Merriam, G. R. (2013). "Macimorelin (AEZS-130)-Stimulated Growth Hormone (GH) Test: Validation of a Novel Oral Stimulation Test for the Diagnosis of Adult GH Deficiency". Journal of Clinical Endocrinology & Metabolism. 98 (6): 2422. doi:10.1210/jc.2013-1157.
This article is issued from Wikipedia - version of the 1/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.