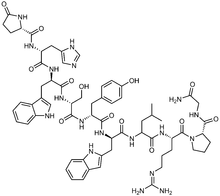
Triptorelin
 | |
| Clinical data | |
|---|---|
| Trade names | Trelstar |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | L02AE04 (WHO) QH01CA97 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
57773-63-4 |
| PubChem (CID) | 25074470 |
| IUPHAR/BPS | 1177 |
| DrugBank |
DB06825 |
| ChemSpider |
17290424 |
| UNII |
9081Y98W2V |
| KEGG |
D06247 |
| ChEBI |
CHEBI:63633 |
| ChEMBL |
CHEMBL1201334 |
| ECHA InfoCard | 100.165.044 |
| Chemical and physical data | |
| Formula | C64H82N18O13 |
| Molar mass | 1311.5 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Triptorelin, a decapeptide (pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2), is a gonadotropin-releasing hormone agonist (GnRH agonist) used as the acetate or pamoate salts. By causing constant stimulation of the pituitary, it decreases pituitary secretion of gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH).
Medical uses
Triptorelin is used to treat prostate cancer.[1]
Names
Triptorelin is marketed under the brand names Decapeptyl (Ipsen) and Diphereline and Gonapeptyl (Ferring Pharmaceuticals). In the United States, it is sold by Watson Pharmaceuticals as Trelstar. In Iran Triptorelin is marketed under the brand name Variopeptyl.
References
- ↑ "triptorelin (Intramuscular route)". drugs.com. Retrieved 11 November 2016.
- Lahlou N, Carel JC, Chaussain JL, Roger M (July 2000). "Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics". J Pediatr Endocrinol Metab. 13 Suppl 1: 723–37. PMID 10969915.
- Padula AM (August 2005). "GnRH analogues—agonists and antagonists". Anim Reprod Sci. 88 (1–2): 115–26. doi:10.1016/j.anireprosci.2005.05.005. PMID 15955640.
This article is issued from Wikipedia - version of the 11/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.