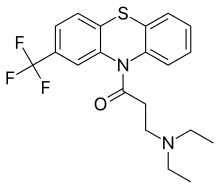
Fluacizine
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, IM |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
30223-48-4 |
| PubChem (CID) | 161562 |
| ChemSpider |
141910 |
| UNII |
E2M3325B1R |
| ChEMBL |
CHEMBL92281 |
| Chemical and physical data | |
| Formula | C20H21F3N2OS |
| Molar mass | 394.455 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Fluacizine (also known as phthorazisin, fluoracizine, and ftoracizine) is a phenothiazine derivative which, unlike most other phenothiazines which are used as antipsychotics, is an antidepressant with sedative effects that was marketed in Russia.[1][2][3][4] It is known to act as a norepinephrine reuptake inhibitor, antihistamine, and anticholinergic.[1][5][6][7] It is no longer used.
References
- 1 2 Author Unknown (1972). Ann Reports Medicinal Chem V7 (v. 7). Boston: Academic Press. ISBN 0-12-040507-5.
- ↑ Vikhlyaev YI, Zherdev VP, Ul'yanova OV (November 1974). "Distribution and action of fluacizine during prolonged administration". Bulletin of Experimental Biology and Medicine. 77 (5): 524–6. PMID 4474896.
- ↑ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ↑ O'Neil, Maryadele J. (2001). The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Rahway, NJ: Merck Research Laboratories. ISBN 0-911910-13-1.
- ↑ Arefolov VA, Panasyuk LV, Raevskii KS, Kostyukov VI (September 1974). "Effect of fluacizine on the uptake of exogenous noradrenalin by the isolated rat vas deferens". Bulletin of Experimental Biology and Medicine. 77 (3): 295–7. doi:10.1007/bf00802484. PMID 4153328.
- ↑ Arefolov VA, Panasyuk LV (November 1974). "Effect of fluacizine on the uptake of exogenous noradrenalin". Bulletin of Experimental Biology and Medicine. 77 (5): 520–3. PMID 4441683.
- ↑ Arefolov VA, Panasiuk LV, Firsov VK (1975). "[Neuromediator content in the synaptic vesicles of rat adrenergic nerves in some pharmacological actions]". Farmakologiia I Toksikologiia (in Russian). 38 (3): 285–9. PMID 6305.
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
Antipsychotics (neuroleptics) (N05A) | |
|---|---|
| Typical |
|
| Atypical |
|
| Others | |
| |
| GABAA |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAB | |||||||||||||||||||||||||||||||||||||||||||
| H1 |
| ||||||||||||||||||||||||||||||||||||||||||
| α2-Adrenergic | |||||||||||||||||||||||||||||||||||||||||||
| 5-HT2A |
| ||||||||||||||||||||||||||||||||||||||||||
| Melatonin | |||||||||||||||||||||||||||||||||||||||||||
| Orexin | |||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
| α1 |
| ||||||
|---|---|---|---|---|---|---|---|
| α2 |
| ||||||
| β |
| ||||||
| |||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (inhibitors) |
| ||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||
| Others |
| ||||||||||||||
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|
This article is issued from Wikipedia - version of the 10/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.