Pagoclone
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
133737-32-3 |
| PubChem (CID) | 131664 |
| ChemSpider |
116335 |
| UNII |
38VAG2SA33 |
| ChEMBL |
CHEMBL2104745 |
| Chemical and physical data | |
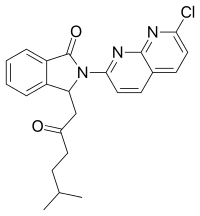
| Formula | C23H22ClN3O2 |
| Molar mass | 407.893 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Pagoclone is an anxiolytic agent from the cyclopyrrolone family, related to better-known drugs such as the sleeping medication zopiclone. It was synthesized by a French team working for Rhone-Poulenc & Rorer S.A.[1] Pagoclone belongs to the class of nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It was never commercialised.
It binds with roughly equivalent high affinity (0.7–9.1 nM) to the benzodiazepine binding site of human GABAA receptors containing either an α1, α2, α3 or α5 subunit. It is a partial agonist at α1-, α2- and α5-containing GABAA receptors and a full agonist at receptors containing an α3 subunit. In rats 5′-hydroxypagoclone was identified as a major metabolite. This metabolite has a considerably greater efficacy at the α1 subtype than the parent compound and was shown to have significant anxiolytic-like activity and to produce sedation.[2][3] In contrast to zopiclone, pagoclone produces anxiolytic effects with little sedative or amnestic actions at low doses.[4]
The pharmacologist David Nutt has suggested pagoclone as a possible base from which to make a better social drug, as it produces the positive effects of alcohol, such as relaxation and sociability, but without also causing the negative effects like aggression, amnesia, nausea, loss of coordination and liver damage. Its effect can be quickly reversed by the action of flumazenil, which is already used as an antidote to benzodiazepine overdose.[5] Nutt has published studies[6] praising the potential of pagoclone which were financed by Indevus which was seeking funding for a possible production of the compound. The long-term safety of pagoclone has not been assessed. The abuse potential of pagoclone has been assessed as being similar to, or slightly less than that of diazepam and it would also be expected to be somewhat safer due to its relatively weaker sedative effects,[7] but development of pagoclone as a commercial drug would still be unlikely due to concerns about abuse.
Pagoclone was trialed as a drug to improve a stammerer's speech fluency,[8] but research for this application was discontinued following disappointing results in Phase II clinical trials.
Synthesis
Pagoclone and pazinaclone are both isoindolone
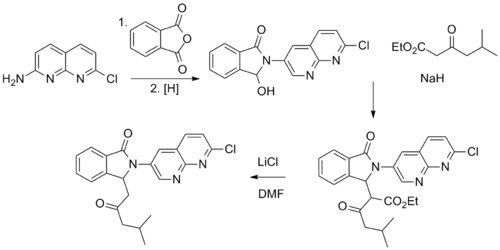
Reaction of 2-Amino-7-chloro-1,8-naphthyridine with phthalic anhydride leads to the corresponding phthalimide. Selective reduction of one of the imide carbonyl groups give the corresponding alcohol. Reaction with the carbanion from Ethyl 5-methyl-3-oxohexanoate leads to the product from the displacement of the hydroxyl group; 'this too may proceed via the acrylate obtained from aldol reaction of the ring opened imidal'.
See also
References
- ↑ 2-amino naphthyridine derivative, its preparation and its use US 5498716
- ↑ Atack JR, Pike A, Marshall G, Stanley J, Lincoln R, Cook SM, Lewis RT, Blackaby WP, Goodacre SC, McKernan RM, Dawson GR, Wafford KA, Reynolds DS (2006). "The in vivo properties of pagoclone in rat are most likely mediated by 5'-hydroxy pagoclone". Neuropharmacology. 50 (6): 677–89. doi:10.1016/j.neuropharm.2005.11.014. PMID 16430927.
- ↑ Atack, JR (May 2005). "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opinion on Investigational Drugs. 14 (5): 601–18. doi:10.1517/13543784.14.5.601. PMID 15926867.
- ↑ Atack, JR (Aug 2003). "Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site". Current Drug Targets. CNS and Neurological Disorders. 2 (4): 213–32. doi:10.2174/1568007033482841. PMID 12871032.
- ↑ Nutt DJ (2006). "For "Critique and Commentaries" section of the Journal of Psychopharmacology: Alcohol alternatives - a goal for psychopharmacology?". Journal of Psychopharmacology. 20 (3): 318–320. doi:10.1177/0269881106063042. PMID 16574703.
- ↑ Lingford-Hughes A; et al. (2005). "A proof-of-concept study using [11C]flumazenil PET to demonstrate that pagoclone is a partial agonist". Psychopharmacology. 180 (4): 1–3. doi:10.1007/s00213-005-0060-1. PMID 15986186.
- ↑ de Wit H, Vicini L, Haig GM, Hunt T, Feltner D. Evaluation of the abuse potential of pagoclone, a partial GABAA agonist. Journal of Clinical Psychopharmacology. 2006 Jun;26(3):268-73.
- ↑ Maguire, G; Franklin, D; Vatakis, NG; Morgenshtern, E; Denko, T; Yaruss, JS; Spotts, C; Davis, L; Davis, A; Fox, P; Soni, P; Blomgren, M; Silverman, A; Riley, G (February 2010). "Exploratory randomized clinical study of pagoclone in persistent developmental stuttering: the EXamining Pagoclone for peRsistent dEvelopmental Stuttering Study.". Journal of Clinical Psychopharmacology. 30 (1): 48–56. doi:10.1097/jcp.0b013e3181caebbe. PMID 20075648.