4-Fluoromethamphetamine
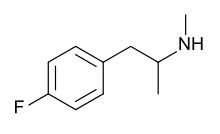
4-Fluoromethamphetamine (4-FMA) is a stimulant drug related to methamphetamine and 4-fluoroamphetamine. It has been reported to be sold on the illicit market as a controlled substance analogue, but little is known about its pharmacology or toxicology as yet.[1] It was first detected from legal highs sold in Japan in 2006 and became illegal to sell or to possess for the purpose of distribution (although not to simply possess for personal use) in Japan in 2008.[2] It was initially reported to be contained as an ingredient in some of the range of party pills sold internationally by the Israeli company Neorganics from around 2006 onwards, but this was later shown to be incorrect and this ingredient was eventually identified as the closely related compound 2-fluoromethamphetamine.[3]
Pharmacology
4-FMA is a CYP450 inhibitor. It reduces the metabolism of methamphetamine, which has the effect of increasing its potency, duration and systemic toxicity while also reducing its cellular toxicity.[4]
Legal Status
China
As of October 2015 4-FMA is a controlled substance in China.[5]
Australia
4-FMA is considered a Schedule 9 substance in Australia under the Poisons Standard (October 2015).[6] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[6]
See also
References
- ↑ Rösner, P.; Quednow, B.; Girreser, U.; Junge, T. (2005). "Isomeric Fluoro-methoxy-phenylalkylamines: A new series of controlled-substance analogues (designer drugs)". Forensic Science International. 148 (2–3): 143–156. doi:10.1016/j.forsciint.2004.05.003. PMID 15639609.
- ↑ Machiko Nagashima; Takako Seto; Misako Takahashi; Jin Suzuki; Ichirou Yasuda (2006). "Spectrum Data of the 3rd Governor-designated Drugs and the Analyses of Uncontrolled Drugs Purchased" (PDF). Ann. Rep. Tokyo Metr. Inst.P.H. 57: 109–113.
- ↑ Camilleri, A; Johnston, MR; Brennan, M; Davis, S; Caldicott, DG (2010). "Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone". Forensic Science International. 197 (1–3): 59–66. doi:10.1016/j.forsciint.2009.12.048. PMID 20074881.
- ↑ Cherner, M.; Bousman, C.; Everall, I. A. N.; Barron, D.; Letendre, S.; Vaida, F.; Atkinson, J. H.; Heaton, R.; Grant, I.; Hnrc, G. (2010). "Cytochrome P450-2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: Preliminary findings". Journal of the International Neuropsychological Society. 16 (5): 890–901. doi:10.1017/S1355617710000779. PMC 3543816
 . PMID 20727252.
. PMID 20727252. - ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- 1 2 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
|
|---|
|
| Adamantanes | |
|---|
|
| Adenosine antagonists | |
|---|
|
| Alkylamines | |
|---|
|
| Ampakines | |
|---|
|
| Arylcyclohexylamines | |
|---|
|
| Benzazepines | |
|---|
|
| Cholinergics | |
|---|
|
| Convulsants | |
|---|
|
| Eugeroics | |
|---|
|
| Oxazolines | |
|---|
|
| Phenethylamines |
- 1-(4-Methylphenyl)-2-aminobutane
- 1-Phenyl-2-(piperidin-1-yl)pentan-3-one
- 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane
- 2-Fuoroamphetamine
- 2-Fuoromethamphetamine
- 2-OH-PEA
- 2-Phenyl-3-aminobutane
- 2-Phenyl-3-methylaminobutane
- 2,3-MDA
- 3-Fuoroamphetamine
- 3-Fluoroethamphetamine
- 3-Fluoromethcathinone
- 3-Methoxyamphetamine
- 3-Methylamphetamine
- 3,4-DMMC
- 4-BMC
- 4-CMC
- 4-Ethylamphetamine
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-MA
- 4-Methylbuphedrone
- 4-Methylcathinone
- 4-MMA
- 4-Methylpentedrone
- 4-MTA
- 6-FNE
- AL-1095
- Alfetamine
- a-Ethylphenethylamine
- Amfecloral
- Amfepentorex
- Amfepramone
- Amidephrine
- 2-Amino-1,2-dihydronaphthalene
- 2-Aminoindane
- 5-(2-Aminopropyl)indole
- 2-Aminotetralin
- Acridorex
- Amphetamine (Dextroamphetamine, Levoamphetamine)
- Amphetaminil
- Arbutamine
- β-Methylphenethylamine
- β-Phenylmethamphetamine
- Benfluorex
- Benzedrone
- Benzphetamine
- BDB
- BOH
- 3-Benzhydrylmorpholine
- BPAP
- Buphedrone
- Bupropion
- Butylone
- Camfetamine
- Cathine
- Cathinone
- Chlorphentermine
- Cilobamine
- Cinnamedrine
- Clenbuterol
- Clobenzorex
- Cloforex
- Clortermine
- Cypenamine
- D-Deprenyl
- Denopamine
- Dimethoxyamphetamine
- Dimethylamphetamine
- Dimethylcathinone
- Dobutamine
- DOPA (Dextrodopa, Levodopa)
- Dopamine
- Dopexamine
- Droxidopa
- EBDB
- Ephedrine
- Epinephrine
- Epinine
- Etafedrine
- Ethcathinone
- Ethylnorepinephrine
- Ethylone
- Etilamfetamine
- Etilefrine
- Famprofazone
- Fencamfamine
- Fencamine
- Fenethylline
- Fenfluramine (Dexfenfluramine, Levofenfluramine)
- Fenproporex
- Feprosidnine
- Flephedrone
- Fludorex
- Formetorex
- Furfenorex
- Gepefrine
- Hexapradol
- Hexedrone
- HMMA
- Hordenine
- 4-Hydroxyamphetamine
- 5-Iodo-2-aminoindane
- Ibopamine
- IMP
- Indanylamphetamine
- Iofetamine
- Isoetarine
- Isoethcathinone
- Isoprenaline
- L-Deprenyl (Selegiline)
- Lefetamine
- Lisdexamfetamine
- Lophophine
- MBDB
- MDA
- MDBU
- MDEA
- MDMA
- MDMPEA
- MDOH
- MDPR
- MDPEA
- Mefenorex
- Mephedrone
- Mephentermine
- Metanephrine
- Metaraminol
- Mesocarb
- Methamphetamine (Dextromethamphetamine, Levomethamphetamine)
- Methoxamine
- Methoxyphenamine
- MMA
- Methcathinone
- Methedrone
- Methoxyphenamine
- Methylenedioxycathinone
- Methylone
- Mexedrone
- MMDA
- MMDMA
- MMMA
- Morforex
- N,alpha-Diethylphenylethylamine
- N-Benzyl-1-phenethylamine
- N-Ethylbuphedrone
- N-Ethylhexedrone
- N,N-Dimethylphenethylamine
- Naphthylamphetamine
- Nisoxetine
- Norepinephrine
- Norfenefrine
- Norfenfluramine
- Normetanephrine
- L-Norpseudoephedrine
- Octopamine (drug)
- Orciprenaline
- Ortetamine
- Oxifentorex
- Oxilofrine
- PBA
- PCA
- PCMA
- PHA
- Pentorex
- Pentedrone
- Pentylone
- Phenatine
- Phenpromethamine
- Phentermine
- Phenylalanine
- Phenylephrine
- Phenylpropanolamine
- Pholedrine
- PIA
- PMA
- PMEA
- PMMA
- PPAP
- Phthalimidopropiophenone
- Prenylamine
- Propylamphetamine
- Pseudoephedrine
- Ropinirole
- Salbutamol (Levosalbutamol)
- Sibutramine
- Synephrine
- Theodrenaline
- Tiflorex
- Tranylcypromine
- Tyramine
- Tyrosine
- Xylopropamine
- Zylofuramine
|
|---|
|
| Phenylmorpholines | |
|---|
|
| Piperazines | |
|---|
|
| Piperidines | |
|---|
|
| Pyrrolidines | |
|---|
|
| Racetams | |
|---|
|
| Tropanes | |
|---|
|
| Tryptamines | |
|---|
|
| Others | |
|---|
|
|
|
|---|
|
| α1 | | |
- Antagonists
- Abanoquil
- Adimolol
- Ajmalicine
- Alfuzosin
- Amosulalol
- Anisodamine
- Arotinolol
- Atiprosin
- Atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, risperidone)
- Benoxathian
- Buflomedil
- Bunazosin
- Carvedilol
- Corynanthine
- Dapiprazole
- Domesticine
- Doxazosin
- Ergolines (e.g., ergotamine, dihydroergotamine, lisuride, terguride)
- Etoperidone
- Eugenodilol
- Fenspiride
- Hydroxyzine
- Indoramin
- Ketanserin
- L-765,314
- Labetalol
- mCPP
- Mepiprazole
- Metazosin
- Monatepil
- Moxisylyte
- Naftopidil
- Nantenine
- Nefazodone
- Neldazosin
- Niaprazine
- Nicergoline
- Niguldipine
- Pardoprunox
- Pelanserin
- Phendioxan
- Phenoxybenzamine
- Phentolamine
- Piperoxan
- Prazosin
- Quinazosin
- Ritanserin
- Silodosin
- Spiperone
- Talipexole
- Tamsulosin
- Terazosin
- Tiodazosin
- Tolazoline
- Trazodone
- Tetracyclic antidepressants (e.g., amoxapine, maprotiline, mianserin)
- Tricyclic antidepressants (e.g., amitriptyline, clomipramine, doxepin, imipramine, trimipramine)
- Trimazosin
- Typical antipsychotics (e.g., chlorpromazine, fluphenazine, loxapine, thioridazine)
- Urapidil
- WB-4101
- Zolertine
|
|
|---|
|
| α2 | | |
- Antagonists
- 1-PP
- Adimolol
- Aptazapine
- Atipamezole
- Atypical antipsychotics (e.g., asenapine, clozapine, lurasidone, paliperidone, quetiapine, risperidone, zotepine)
- Azapirones (e.g., buspirone, tandospirone)
- BRL-44408
- Buflomedil
- Cirazoline
- Efaroxan
- Esmirtazapine
- Fenmetozole
- Fluparoxan
- Idazoxan
- mCPP
- Mianserin
- Mirtazapine
- NAN-190
- Olanzapine
- Pardoprunox
- Phentolamine
- Phenoxybenzamine
- Piperoxan
- Piribedil
- Rauwolscine
- Rotigotine
- SB-269970
- Setiptiline
- Spiroxatrine
- Sunepitron
- Tolazoline
- Typical antipsychotics (e.g., chlorpromazine, fluphenazine, loxapine, thioridazine)
- Yohimbine
|
|
|---|
|
| β | |
|---|
|
- See also: Dopaminergics
- Melatonergics
- Serotonergics
- Monoamine reuptake and release modulators
- Monoamine metabolism modulators
- Monoamine neurotoxins
|
 . PMID 20727252.
. PMID 20727252.