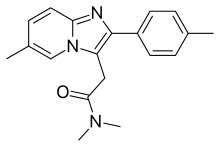
Zolpidem
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | originally Ambien, many names worldwide[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693025 |
| Pregnancy category | |
| Dependence liability | High |
| Routes of administration | Oral (tablet), sublingual, oromucosal (spray), rectal |
| ATC code | N05CF02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70% (oral) |
| Protein binding | 92% |
| Metabolism | Liver through CYP3A4 |
| Biological half-life | 2–3 hours |
| Duration of action | 3 hours |
| Excretion |
Kidney (56%) fecal (34%) |
| Identifiers | |
| |
| CAS Number |
82626-48-0 |
| PubChem (CID) | 5732 |
| IUPHAR/BPS | 4362 |
| DrugBank |
DB00425 |
| ChemSpider |
5530 |
| UNII |
7K383OQI23 |
| KEGG |
D08690 |
| ChEBI |
CHEBI:10125 |
| ChEMBL |
CHEMBL911 |
| Chemical and physical data | |
| Formula | C19H21N3O |
| Molar mass | 307.395 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Zolpidem (originally marketed as Ambien and available worldwide under many brand names)[1] is a sedative primarily used for the treatment of insomnia. It works quickly, usually within 15 minutes, and has a short half-life of two to three hours. Zolpidem has not adequately demonstrated effectiveness in maintaining sleep, unless delivered in a controlled-release (CR) form. However, it is effective in initiating sleep.[2] Its hypnotic effects are similar to those of the benzodiazepine class of drugs.
In 2013, the Food and Drug Administration required manufacturers to decrease the recommended dose for women by half, after studies showed that the medicines can leave people drowsy in the morning and at risk for motor vehicle collisions. The FDA recommended that manufacturers extend the new dosage cuts to men as well, who process the drug at a faster rate; however, the reasons men and women metabolize the drugs at different rates are still unknown.[3] In May 2013, the FDA approved label changes specifying new dosage recommendations for zolpidem products because of concerns regarding next-morning impairment.[4] The underlying mechanism involves GABA.
It is a short-acting nonbenzodiazepine compound of the imidazopyridine class[5] that increases the activity of GABA, an inhibitory neurotransmitter, by binding to GABAA receptors at the same location as benzodiazepines.[6] It is molecularly distinct from the classical benzodiazepine molecule and is classified as an imidazopyridine. Flumazenil, a benzodiazepine receptor antagonist, which is used for benzodiazepine overdose, can also reverse zolpidem's sedative/hypnotic and memory-impairing effects.[7][8]
The United States patent for zolpidem was held by the French pharmaceutical corporation Sanofi-Aventis.[9] On April 23, 2007, the U.S. Food and Drug Administration (FDA) approved 13 generic versions of zolpidem tartrate.[10] Zolpidem is available from several generic manufacturers in the UK, as a generic from Sandoz in South Africa and TEVA in Israel, as well as from other manufacturers such as Ratiopharm and Takeda GmbH (both Germany).
Medical uses

Sleep
Clinicians prescribe zolpidem for short-term (usually about two to six weeks) treatment of insomnia.[11] Zolpidem addresses sleep-initiation problems, but is not effective in maintaining sleep.[2] Also, a 2012 NIH study showed that zolpidem's effectiveness is nearly as much due to psychological effects as to the drug itself, so "increased attention should be directed at psychological intervention of insomnia."[12]
Other
Zolpidem has some muscle relaxant and anticonvulsant properties, but has not been approved for use in muscle relaxation or seizure prevention. This is because the dosage of drug needed to cause muscle relaxation is 10 times the sedating dose, while early studies indicated that the dosage needed for preventing seizures is 20 times the sedating dose.[13]
Adverse effects
The most common side effects for short-term use include headache (reported by 7% of people in clinical trials) drowsiness (2%), dizziness (1%), and diarrhea (1%); the most common side effects of long-term use included dry mouth (3%), allergy (4%), back pain (3%), flu-like symptoms (1%), chest pain (1%), heart palpitations (2%), drowsiness (8%), dizziness (5%), lethargy (3%), drugged feeling (3%), lightheadedness (2%), depression (1%), abnormal dreams (1%), amnesia (1%), sleep disorder (1%), diarrhea (3%), abdominal pain (2%), constipation (2%), sinusitis (4%), sore throat (3%), and rash (2%).[5]
Some users have reported unexplained sleepwalking[14] while using zolpidem, as well as sleep driving,[14] Night eating syndrome while asleep, and performing other daily tasks while sleeping. Research by Australia's National Prescribing Service found these events occur mostly after the first dose taken, or within a few days of starting therapy.[15] Rare reports of sexual parasomnia episodes related to zolpidem intake have also been reported.[16] Sleepwalkers can sometimes perform these tasks as normally as they might if they were awake.
Residual 'hangover' effects, such as sleepiness and impaired psychomotor and cognitive function, may persist into the day following nighttime administration. Such effects may impair the ability of users to drive safely and increase risks of falls and hip fractures.[14][17]
In February 2008, the Australian Therapeutic Goods Administration attached a boxed warning to zolpidem, stating that "Zolpidem may be associated with potentially dangerous complex sleep-related behaviors that may include sleep walking, sleep driving, and other bizarre behaviours. Zolpidem is not to be taken with alcoholic beverages. Caution is needed with other CNS-depressant drugs. Limit use to four weeks maximum under close medical supervision."[18]
Tolerance, dependence, and withdrawal
A review medical publication found long-term use of zolpidem is associated with drug tolerance, substance dependence, rebound insomnia, and CNS-related adverse effects. It was recommended that zolpidem be used for short periods of time using the lowest effective dose. Zolpidem 10 mg is effective in treating insomnia when used intermittently no fewer than three and no more than five pills per week for a period of 12 weeks.[19] The 15-mg zolpidem dosage provided no clinical advantage over the 10-mg zolpidem dosage.[20]
Nonpharmacological treatment options (e.g. cognitive behavioral therapy for insomnia), however, were found to have sustained improvements in sleep quality.[21] Animal studies of the tolerance-inducing properties have shown that in rodents, zolpidem has less tolerance-producing potential than benzodiazepines, but in primates the tolerance-producing potential of zolpidem was the same as that of benzodiazepines.[22] Tolerance to the effects of zolpidem can develop in some people in just a few weeks. Abrupt withdrawal may cause delirium, seizures, or other severe effects, especially if used for prolonged periods and at high dosages.[23][24][25]
When drug tolerance and physical dependence to zolpidem has developed, treatment usually entails a gradual dose reduction over a period of months to minimise withdrawal symptoms, which can resemble those seen during benzodiazepine withdrawal. Failing that, an alternative method may be necessary for some patients, such as a switch to a benzodiazepine equivalent dose of a longer-acting benzodiazepine drug, such as diazepam or chlordiazepoxide, followed by a gradual reduction in dosage of the long-acting benzodiazepine. Sometimes for difficult-to-treat patients, an inpatient flumazenil rapid detoxification program can be used to detoxify from a zolpidem drug dependence or addiction.[26]
Alcohol has cross tolerance with GABAA receptor positive modulators such as the benzodiazepines and the nonbenzodiazepine drugs. For this reason, alcoholics or recovering alcoholics may be at increased risk of physical dependency on zolpidem. Also, alcoholics and drug abusers may be at increased risk of abusing and or becoming psychologically dependent on zolpidem. It should be avoided in those with a history of alcoholism, drug misuse, physical dependency, or psychological dependency on sedative-hypnotic drugs. Zolpidem has rarely been associated with drug-seeking behavior, the risk of which is amplified in patients with a history of drug or alcohol abuse.
Overdose
An overdose of zolpidem may cause excessive sedation, pin-point pupils, or depressed respiratory function, which may progress to coma, and possibly death. Combined with alcohol, opiates, or other CNS depressants, it may be even more likely to lead to fatal overdoses. Zolpidem overdosage can be treated with the benzodiazepine receptor antagonist flumazenil, which displaces zolpidem from its binding site on the benzodiazepine receptor to rapidly reverse the effects of the zolpidem.[27]
Detection in body fluids
Zolpidem may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients, provide evidence in an impaired driving arrest, or to assist in a medicolegal death investigation. Blood or plasma zolpidem concentrations are usually in a range of 30–300 μg/l in persons receiving the drug therapeutically, 100–700 μg/l in those arrested for impaired driving, and 1000–7000 μg/l in victims of acute overdosage. Analytical techniques, in general, involve gas or liquid chromatography.[28][29][30]
Special precautions
Driving
Use of zolpidem may impair driving skills with a resultant increased risk of road traffic accidents. This adverse effect is not unique to zolpidem but also occurs with other hypnotic drugs. Caution should be exercised by motor vehicle drivers.[31] Studies showed that eight hours after a bedtime dose of 10 mg, 15% of women and 3% of men would have blood levels that produce impaired driving skills; for an extended-release dose of 12.5 mg, the risk increased to 33% and 25%, respectively. As a consequence, the FDA recommended the dose for women be reduced and that prescribers should consider lower doses for men.[32][33]
Elderly
The elderly are more sensitive to the effects of hypnotics including zolpidem. Zolpidem causes an increased risk of falls and may induce adverse cognitive effects.[34]
An extensive review of the medical literature regarding the management of insomnia and the elderly found that there is considerable evidence of the effectiveness and durability of nondrug treatments for insomnia in adults of all ages, and these interventions are underused. Compared with the benzodiazepines, the nonbenzodiazepine (including zolpidem) sedative-hypnotics appeared to offer few, if any, significant clinical advantages in efficacy or tolerability in elderly persons. Newer agents with novel mechanisms of action and improved safety profiles, such as the melatonin receptor agonists, were found to hold promise for the management of chronic insomnia in elderly people.
Long-term use of sedative-hypnotics for insomnia lacks an evidence base and has traditionally been discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment (anterograde amnesia), daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls. In addition, the effectiveness and safety of long-term use of these agents remain to be determined. More research is needed to evaluate the long-term effects of treatment and the most appropriate management strategy for elderly persons with chronic insomnia.[35]
Gastroesophageal reflux disease
Patients suffering from gastroesophageal reflux disease (GERD) had reflux events measured to be significantly longer when taking zolpidem than on placebo. The same trend was found for reflux events in patients without GERD. This is assumed to be due to suppression of arousal during the reflux event, which would normally result in a swallowing reflex to clear gastric acid from the esophagus. Patients with GERD experience significantly higher esophageal exposure to gastric acid, which increases the likelihood of their developing esophageal cancer.[36]
Pregnancy
Zolpidem has been assigned to pregnancy category C by the FDA. Animal studies have revealed evidence of incomplete ossification and increased postimplantation fetal loss at doses greater than seven times the maximum recommended human dose or higher; however, teratogenicity was not observed at any dose level. There are no controlled data in human pregnancy. In one case report, zolpidem was found in cord blood at delivery. Zolpidem is recommended for use during pregnancy only when benefits outweigh risks. [37]
Mechanism of action

Zaleplon and zolpidem both are agonists at the GABA A α 1 subunit. Due to its selective binding, zolpidem has very weak anxiolytic, myorelaxant, and anticonvulsant properties but very strong hypnotic properties.[38] Zolpidem binds with high affinity and acts as a full agonist at the α1-containing GABAA receptors, about 10-fold lower affinity for those containing the α2- and α3- GABAA receptor subunits, and with no appreciable affinity for α5 subunit-containing receptors.[39][40] ω1 type GABAA receptors are the α1-containing GABAA receptors and ω2 GABAA receptors are the α2-, α3-, α4-, α5-, and α6-containing GABAA receptors. ω1 GABAA receptors are found primarily in the brain, whereas ω2 receptors are found primarily in the spine. Thus, zolpidem has a preferential binding for the GABAA-benzodiazepine receptor complex in the brain but a low affinity for the GABAA-benzodiazepine receptor complex in the spine.[41]
Like the vast majority of benzodiazepine-like molecules, zolpidem has no affinity for α4 and α6 subunit-containing receptors.[42] Zolpidem positively modulates GABAA receptors, it is presumed by increasing the GABAA receptor complex's apparent affinity for GABA without affecting desensitization or peak current.[43] Like zaleplon (Sonata), zolpidem may increase slow wave sleep but cause no effect on stage 2 sleep.[44]
A meta-analysis of the randomised, controlled, clinical trials that compared benzodiazepines against nonbenzodiazepines such as zolpidem has shown few consistent differences between zolpidem and benzodiazepines in terms of sleep onset latency, total sleep duration, number of awakenings, quality of sleep, adverse events, tolerance, rebound insomnia, and daytime alertness.[45]
Chemistry
Three syntheses of zolpidem are common. 4-methylacetophenone is used as a common precursor. This is brominated and reacted with 2-amino-5-methylpyridine to give the imidazopyridine. From here the reactions use a variety of reagents to complete the synthesis, either involving thionyl chloride or sodium cyanide. These reagents are challenging to handle and require thorough safety assessments.[46][47][48] Though such safety procedures are common in industry, they make clandestine manufacture difficult.
A number of major side-products of the sodium cyanide reaction have been characterised and include dimers and mannich products.[49]
Drug–drug interactions
Notable drug–drug interactions with the pharmacokinetics of zolpidem include chlorpromazine, fluconazole, imipramine, itraconazole, ketoconazole, rifampicin, and ritonavir. Interactions with carbamazepine and phenytoin can be expected based on their metabolic pathways, but have not yet been studied. There does not appear to be any interaction between zolpidem and cimetidine or ranitidine.[50][51] However, it was noted in the same study that cimetidine did appear to prolong the hypnotic effects of Zolpidem beyond its typical 3 hour duration, which is indicative of some sort of metabolic interaction.[50]
Usage
Zolpidem is one of the most common GABA-potentiating sleeping medications prescribed in the Netherlands, with a total of 582,660 prescriptions dispensed in 2008.[52]
The United States Air Force uses zolpidem as one of the hypnotics approved as a "no-go pill" (with a 6-hour restriction on subsequent flight operation) to help aviators and special duty personnel sleep in support of mission readiness. (The other hypnotics used are temazepam and zaleplon.) "Ground tests" are required prior to authorization issued to use the medication in an operational situation.[53]
Society and culture
Abuse
Zolpidem has a potential for either medical misuse when the drug is continued long term without or against medical advice or recreational use when the drug is taken to achieve a "high".[54] The transition from medical use of zolpidem to high-dose addiction or drug dependence can occur when used without a doctor's recommendation to continue using it, when physiological drug tolerance leads to higher doses than the usual 5 mg or 10 mg, when consumed through inhalation or injection, or when taken for purposes other than as a sleep aid. Misuse is more prevalent in those having been dependent on other drugs in the past, but tolerance and drug dependence can still sometimes occur in those without a history of drug dependence. Chronic users of high doses are more likely to develop physical dependence on the drug, which may cause severe withdrawal symptoms, including seizures, if abrupt withdrawal from zolpidem occurs.[55]
One case history reported a woman detoxifying from a high dose of zolpidem experiencing a generalized seizure, with clinical withdrawal and dependence effects reported to be similar to the benzodiazepine withdrawal syndrome.[56]
Other drugs, including the benzodiazepines and zopiclone, are also found in high numbers of suspected drugged drivers. Many drivers have blood levels far exceeding the therapeutic dose range suggesting a high degree of excessive-use potential for benzodiazepines, zolpidem and zopiclone.[57] U.S. Congressman Patrick J. Kennedy says that he was using Zolpidem (Ambien) and Phenergan when caught driving erratically at 3AM.[58] "I simply do not remember getting out of bed, being pulled over by the police, or being cited for three driving infractions," Kennedy said.
Nonmedical use of zolpidem is increasingly common in the U.S., Canada, and the UK. Recreational users report that resisting the drug's hypnotic effects can in some cases elicit vivid visuals and a body high.[59] Some users have reported decreased anxiety, mild euphoria, perceptual changes, visual distortions, and hallucinations.[60]
Zolpidem (Stilnox) was used by Australian Olympic swimmers at the London Olympics in 2012, leading to controversy.[61]
Regulation
Zolpidem, along with the other benzodiazepine-like Z-drugs, is a Schedule IV controlled substance in the U.S., according to the Controlled Substances Act, given its potential for abuse and dependence.
Date rape drug
Zolpidem has become one of many date rape drugs.[62][63] Unlike Rohypnol ("roofies"), which was banned in 1996, zolpidem is available legally by prescription, and unlike gamma-hydroxybutyrate, which is used to treat a rare form of narcolepsy, zolpidem was prescribed 43.8 million times in the U.S. in 2012.[62] It dissolves readily in liquids such as wine,[62] and can typically be detected in bodily fluids for only 36 hours, though it may be possible to detect it by hair testing much later;[62] this is due to the short elimination half-life of 2.5–3 hours.[64] This application of the drug was highlighted during proceedings against Darren Sharper, who was accused of using the tablets he was prescribed to facilitate a series of rapes.[62][63]
Sleepwalking
Zolpidem received widespread media coverage in Australia after the death of a student who fell 20 m from the Sydney Harbour Bridge while under the influence of zolpidem.[65]
Research
Zolpidem may provide short-lasting but effective improvement in symptoms of aphasia present in some survivors of stroke. The mechanism for improvement in these cases remains unexplained and is the focus of research by several groups, to explain how a drug that acts as a hypnotic-sedative in people with normal brain function, can increase speech ability in people recovering from severe brain injury. As of 2011 use of zolpidem for this application remains experimental,and is not officially approved by any pharmaceutical manufacturers of zolpidem or medical regulatory agencies worldwide.[66][67][68][69][70]
Zolpidem has been studied to determine whether it causes improved responsiveness or regional cerebral perfusion in patients with persistent vegetative states.[71][72][73][74]
The data suggest that the treatment shows a good response rate amongst patients whose brain damage was caused by stroke and also that the correlation between severity of the damage and likelihood of response to the drug is negative, being approximately 25% amongst lesser brain injury patients, whilst severely brain-damaged patients, such as those that are also in vegetative states, show a response of approximately 5% to 10%. However this trend in response does not appear to have any correlation with the effectiveness of the drug across different levels of injury severity.[75][76][77][78][79]
References
- 1 2 Drugs.com International listings for zolpidem Page accessed Feb 23, 2016
- 1 2 Rosenberg RP (2006). "Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies". Ann Clin Psychiatry. 18 (1): 49–56. doi:10.1080/10401230500464711. PMID 16517453.
- ↑ "FDA tells drugmakers to lower doses for Ambien, other sleeping pills". CBS News. Retrieved 10 January 2013.
- ↑ "FDA Changes Dosing on Ambien, Ambien CR, Zolpidem and Edluar". Lawyersandsettlements.com. 2013-05-15. Retrieved 2014-01-31.
- 1 2 "US Label" (PDF). FDA. August 2016. Retrieved 2016-09-30. See FDA label index page for updates
- ↑ Lemmer B (2007). "The sleep-wake cycle and sleeping pills". Physiol. Behav. 90 (2–3): 285–93. doi:10.1016/j.physbeh.2006.09.006. PMID 17049955.
- ↑ Patat A, Naef MM, van Gessel E, Forster A, Dubruc C, Rosenzweig P (October 1994). "Flumazenil antagonizes the central effects of zolpidem, an imidazopyridine hypnotic". Clin Pharmacol Ther. 56 (4): 430–6. doi:10.1038/clpt.1994.157. PMID 7955804.
- ↑ Wesensten NJ, Balkin TJ, Davis HQ, Belenky GL (September 1995). "Reversal of triazolam- and zolpidem-induced memory impairment by flumazenil". Psychopharmacology (Berl). 121 (2): 242–9. doi:10.1007/BF02245635. PMID 8545530.
- ↑ US 4382938, Kaplan J-P, George P, "Imidazo[1,2-a] pyridine derivatives and their application as pharmaceuticals", published 1983-05-10, issued 1984-07-17, assigned to Synthelabo
- ↑ "FDA Approves First Generic Versions of Ambien (Zolpidem Tartrate) for the Treatment of Insomnia". Retrieved 2010-01-24.
- ↑ "Ambien". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ↑ Huedo-Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN (Dec 17, 2012). "Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration". BMJ (Clinical research ed.). 345: e8343. doi:10.1136/bmj.e8343. PMC 3544552
 . PMID 23248080.
. PMID 23248080.
- ↑ Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, Bartholini G (1986). "Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects". J. Pharmacol. Exp. Ther. 237 (2): 649–58. PMID 2871178.
- 1 2 3 Gunja N (2013). "In the Zzz zone: the effects of Z-drugs on human performance and driving". J Med Toxicol. 9 (2): 163–71. doi:10.1007/s13181-013-0294-y. PMC 3657033
 . PMID 23456542.
. PMID 23456542. - ↑ NPS Position Statement: Zolpidem and sleep-related behaviours (2008). Available at http://nps.org.au/news_and_media/media_releases/repository/zolpidem_stilnox_info_for_prescribers
- ↑ Schenck CH, Arnulf I, Mahowald MW (2007). "Sleep and sex: what can go wrong? A review of the literature on sleep related disorders and abnormal sexual behaviors and experiences". Sleep. 30 (6): 683–702. PMC 1978350
 . PMID 17580590.
. PMID 17580590. - ↑ Vermeeren A (2004). "Residual effects of hypnotics: epidemiology and clinical implications". CNS Drugs. 18 (5): 297–328. doi:10.2165/00023210-200418050-00003. PMID 15089115.
- ↑ Australian Government. "Zolpidem ("Stilnox") – updated information – February 2008". www.tga.gov.au. Archived from the original on 2007-08-12. Retrieved 2009-06-22.
- ↑ Perlis ML, McCall WV, Krystal AD, Walsh JK (2004). "Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia". The Journal of Clinical Psychiatry. 65 (8): 1128–1137. doi:10.4088/jcp.v65n0816. PMID 15323600.
- ↑ Scharf MB, Roth T, Vogel GW, Walsh JK (1994). "A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia". The Journal of Clinical Psychiatry. 55 (5): 192–199. PMID 8071269.
- ↑ Kirkwood CK (1999). "Management of insomnia". J Am Pharm Assoc (Wash). 39 (5): 688–96; quiz 713–4. PMID 10533351.
- ↑ Petroski RE, Pomeroy JE, Das R, Bowman H, Yang W, Chen AP, Foster AC (April 2006). "Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors" (PDF). J. Pharmacol. Exp. Ther. 317 (1): 369–77. doi:10.1124/jpet.105.096701. PMID 16399882.
- ↑ Harter C, Piffl-Boniolo E, Rave-Schwank M (November 1999). "[Development of drug withdrawal delirium after dependence on zolpidem and zoplicone]" [Development of drug withdrawal delirium after dependence on zolpidem and zoplicone]. Psychiatr Prax (in German). 26 (6): 309. PMID 10627964.
- ↑ "Hypnotic dependence: zolpidem and zopiclone too". Prescrire Int. 10 (51): 15. February 2001. PMID 11503851.
- ↑ Sethi PK, Khandelwal DC (February 2005). "Zolpidem at supratherapeutic doses can cause drug abuse, dependence and withdrawal seizure" (PDF). J Assoc Physicians India. 53: 139–40. PMID 15847035.
- ↑ Quaglio G, Lugoboni F, Fornasiero A, Lechi A, Gerra G, Mezzelani P (September 2005). "Dependence on zolpidem: two case reports of detoxification with flumazenil infusion". Int Clin Psychopharmacol. 20 (5): 285–7. doi:10.1097/01.yic.0000166404.41850.b4. PMID 16096519.
- ↑ Lheureux P, Debailleul G, De Witte O, Askenasi R (1990). "Zolpidem intoxication mimicking narcotic overdose: response to flumazenil". Human & Experimental Toxicology. 9 (2): 105–7. doi:10.1177/096032719000900209. PMID 2111156.
- ↑ Jones AW, Holmgren A, Kugelberg FC (2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Ther. Drug Monit. 29 (2): 248–260. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081.
- ↑ Gock SB, Wong SH, Nuwayhid N, Venuti SE, Kelley PD, Teggatz JR, Jentzen JM (1999). "Acute zolpidem overdose—report of two cases". J. Anal. Toxicol. 23 (6): 559–562. doi:10.1093/jat/23.6.559. PMID 10517569.
- ↑ R. Baselt (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 1836–1838.
- ↑ Gustavsen I, Bramness JG, Skurtveit S, Engeland A, Neutel I, Mørland J (December 2008). "Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam". Sleep Med. 9 (8): 818–22. doi:10.1016/j.sleep.2007.11.011. PMID 18226959.
- ↑ "FDA Requires Lower Dosing of Zolpidem". The Medical Letter on Drugs and Therapeutics. The Medical Letter. 55 (1408): 5. January 21, 2013. Retrieved April 14, 2013.
- ↑ "FDA Drug Safety Communication: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist)". FDA. January 10, 2013. Retrieved April 14, 2013
- ↑ Antai-Otong D (August 2006). "The art of prescribing. Risks and benefits of non-benzodiazepine receptor agonists in the treatment of acute primary insomnia in older adults". Perspect Psychiatr Care. 42 (3): 196–200. doi:10.1111/j.1744-6163.2006.00070.x. PMID 16916422.
- ↑ Bain KT (June 2006). "Management of chronic insomnia in elderly persons". Am J Geriatr Pharmacother. 4 (2): 168–92. doi:10.1016/j.amjopharm.2006.06.006. PMID 16860264.
- ↑ Gagliardi GS, Shah AP, Goldstein M, Denua-Rivera S, Doghramji K, Cohen S, Dimarino AJ (September 2009). "Effect of zolpidem on the sleep arousal response to nocturnal esophageal acid exposure". Clin. Gastroenterol. Hepatol. 7 (9): 948–52. doi:10.1016/j.cgh.2009.04.026. PMID 19426833.
- ↑ Drugsdb.eu. "Zolpidem Pregnancy Warnings". Retrieved 2014-02-01.
- ↑ Salvà P, Costa J (September 1995). "Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications". Clin Pharmacokinet. 29 (3): 142–53. doi:10.2165/00003088-199529030-00002. PMID 8521677.
- ↑ Pritchett DB, Seeburg PH (1990). "Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology". J. Neurochem. 54 (5): 1802–4. doi:10.1111/j.1471-4159.1990.tb01237.x. PMID 2157817.
- ↑ Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR (2001). "Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux" (PDF). Mol. Pharmacol. 59 (5): 1108–18. PMID 11306694.
- ↑ Rowlett JK, Woolverton WL (November 1996). "Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies". Psychopharmacology (Berl.). 128 (1): 1–16. doi:10.1007/s002130050103. PMID 8944400. Archived from the original on January 12, 2002.
- ↑ Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ (1996). "Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit". Mol. Pharmacol. 50 (3): 670–8. PMID 8794909.
- ↑ Perrais D, Ropert N (1999). "Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses". J. Neurosci. 19 (2): 578–88. PMID 9880578.
- ↑ Noguchi H, Kitazumi K, Mori M, Shiba T (2004). "Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats". J. Pharmacol. Sci. 94 (3): 246–51. doi:10.1254/jphs.94.246. PMID 15037809.
WARNING: The reference indicates that zaleplon-Sonata, not zolpidem, increases Slow-wave sleep
- ↑ Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T (2004). "Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis". Human psychopharmacology. 19 (5): 305–22. doi:10.1002/hup.594. PMID 15252823.
- ↑ Johnson DS, Li JJ (2007). The art of drug synthesis. Hoboken, N.J.: Wiley-Interscience. pp. Chapter 15, Section 2. ISBN 9780471752158.
- ↑ IN 246080, Rawalnath, Sakhardande Rajiv; Richard Crasta Santosh & ALOK SAXENA, "Process for the preparation of zolpidem", published 21 Dec 2005, issued 14-Feb-2011
- ↑ Sumalatha, Y. (2009). "A simple and efficient synthesis of hypnotic agent, zolpidem and its related substances". Arkivoc. 2009 (2): 315–320. doi:10.3998/ark.5550190.0010.230.
- ↑ Sumalatha, Y. (2009). "Synthesis and spectral characterization of zolpidem related substances - hypnotic agent". Arkivoc. 2009 (7): 143–149. doi:10.3998/ark.5550190.0010.714.
- 1 2 Hulhoven R, Desager JP, Harvengt C, Hermann P, Guillet P, Thiercelin JF; Desager; Harvengt; Hermann; Guillet; Thiercelin (1988). "Lack of interaction between zolpidem and H2 antagonists, cimetidine and ranitidine". International journal of clinical pharmacology research. 8 (6): 471–476. PMID 3253224.
- ↑ Wang JS, DeVane CL; Devane (2003). "Pharmacokinetics and drug interactions of the sedative hypnotics" (PDF). Psychopharmacol Bull. 37 (1): 10–29. doi:10.1007/BF01990373. PMID 14561946. Archived from the original (PDF) on 2007-07-09.
- ↑ "Home | GIPdatabank". Gipdatabank.nl. 2013-11-15. Retrieved 2014-01-31.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on June 11, 2014. Retrieved March 8, 2014.
- ↑ Griffiths RR, Johnson MW; Johnson (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry. 66 Suppl 9: 31–41. PMID 16336040.
- ↑ Barrero-Hernández FJ, Ruiz-Veguilla M, López-López MI, Casado-Torres A; Ruiz-Veguilla; López-López; Casado-Torres (2002). "[Epileptic seizures as a sign of abstinence from chronic consumption of zolpidem]" [Epileptic seizures as a sign of abstinence from chronic consumption of zolpidem]. Rev Neurol (in Spanish). 34 (3): 253–6. PMID 12022074.
- ↑ Cubała WJ, Landowski J; Landowski (2007). "Seizure following sudden zolpidem withdrawal". Prog. Neuropsychopharmacol. Biol. Psychiatry. 31 (2): 539–40. doi:10.1016/j.pnpbp.2006.07.009. PMID 16950552.
- ↑ Jones AW, Holmgren A, Kugelberg FC; Holmgren; Kugelberg (2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Therapeutic drug monitoring. 29 (2): 248–60. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081.
- ↑ "Kennedy To Enter Drug Rehab After Car Crash; Congressman Wrecked Car Near Capitol".
- ↑ http://cbs11tv.com/investigators/Ambien.Sleeping.Medication.2.496959.html. Missing or empty
|title=(help)(contains additional text) - ↑ Kim. "ksl.com – Ambien Abuse on Rise Among Teens". www.ksl.com. Retrieved 2009-06-22.
- ↑ "Swimming Australia's 'Stilnox six' given final warning as AOC decides not to issue any further sanctions". www.abc.net.au. Retrieved 2016-08-03.
- 1 2 3 4 5 Brent Schrotenboer (2014-03-26). "Darren Sharper case spotlights sleep drug's dark side". USA Today.
- 1 2 Christian Red (2014-02-17). "In the rape case against Darren Sharper, former LAPD detective says Ambien is used often and can be similar to GHB". New York Daily News.
- ↑ Naren Gunja (June 2013). "The Clinical and Forensic Toxicology of Z-drugs". J Med Toxicol. 9 (2): 155–162. doi:10.1007/s13181-013-0292-0. PMC 3657020
 . PMID 23404347.
. PMID 23404347. - ↑ "Stilnox blamed for Harbour Bridge death". nineMSN News. February 23, 2007. Archived from the original on 2007-06-15.
- ↑ Clauss R, Nel W; Nel (2006). "Drug induced arousal from the permanent vegetative state". NeuroRehabilitation. 21 (1): 23–8. PMID 16720934.
- ↑ Whyte J, Myers R; Myers (May 2009). "Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: a preliminary placebo controlled trial". American Journal of Physical Medicine & Rehabilitation. 88 (5): 410–8. doi:10.1097/PHM.0b013e3181a0e3a0. PMID 19620954.
- ↑ Hall SD, Yamawaki N, Fisher AE, Clauss RP, Woodhall GL, Stanford IM; Yamawaki; Fisher; Clauss; Woodhall; Stanford (April 2010). "GABA(A) alpha-1 subunit mediated desynchronization of elevated low frequency oscillations alleviates specific dysfunction in stroke--a case report". Clinical Neurophysiology. 121 (4): 549–55. doi:10.1016/j.clinph.2009.11.084. PMID 20097125.
- ↑ Nyakale NE, Clauss RP, Nel W, Sathekge M; Clauss; Nel; Sathekge (2010). "Clinical and brain SPECT scan response to zolpidem in patients after brain damage". Arzneimittel-Forschung. 60 (4): 177–81. doi:10.1055/s-0031-1296269. PMID 20486466.
- ↑ Machado C, Estévez M, Pérez-Nellar J, Gutiérrez J, Rodríguez R, Carballo M, Chinchilla M, Machado A, Portela L, García-Roca MC, Beltrán C; Estévez; Pérez-Nellar; Gutiérrez; Rodríguez; Carballo; Chinchilla; Machado; Portela; García-Roca; Beltrán (March 2011). "Autonomic, EEG, and behavioral arousal signs in a PVS case after Zolpidem intake". The Canadian Journal of Neurological Sciences. Le Journal Canadien Des Sciences Neurologiques. 38 (2): 341–4. PMID 21320843.
- ↑ Snyman N, Egan JR, London K, Howman-Giles R, Gill D, Gillis J, Scheinberg A; Egan; London; Howman-Giles; Gill; Gillis; Scheinberg (2011). "Zolpidem for Persistent Vegetative State - A Placebo-Controlled Trial in Pediatrics". Neuropediatrics. 41 (5): 223–227. doi:10.1055/s-0030-1269893. PMID 21210338.
- ↑ Whyte J, Myers R; Myers (2009). "Incidence of Clinically Significant Responses to Zolpidem Among Patients with Disorders of Consciousness". American Journal of Physical Medicine & Rehabilitation. 88 (5): 410–418. doi:10.1097/PHM.0b013e3181a0e3a0. PMID 19620954.
- ↑ Interlandi, Jeneen (December 1, 2011). "A Drug That Wakes the Near Dead". New York Times.
- ↑ Du B, Shan A, Zhang Y, Zhong X, Chen D, Cai K (2014). "Zolpidem Arouses Patients in Vegetative State After Brain Injury". The American Journal of the Medical Sciences. 347 (3): 178–82. doi:10.1097/MAJ.0b013e318287c79c. PMID 23462249.
- ↑ Qin P; Wu X; Duncan NW; Bao W; Tang W; Zhang Z; et al. (2015). "GABAA receptor deficits predict recovery in patients with disorders of consciousness: A preliminary multimodal [(11) C]Flumazenil PET and fMRI study.". Hum Brain Mapp. 36 (10): 3867–77. doi:10.1002/hbm.22883. PMID 26147065.
- ↑ Chatelle C; Thibaut A; Gosseries O; Bruno MA; Demertzi A; Bernard C; et al. (2014). "Changes in cerebral metabolism in patients with a minimally conscious state responding to zolpidem.". Front Hum Neurosci. 8: 917. doi:10.3389/fnhum.2014.00917. PMC 4251320
 . PMID 25520636.
. PMID 25520636. - ↑ Whyte J; Rajan R; Rosenbaum A; Katz D; Kalmar K; Seel R; et al. (2014). "Zolpidem and restoration of consciousness.". Am J Phys Med Rehabil. 93 (2): 101–13. doi:10.1097/PHM.0000000000000069. PMID 24434886.
- ↑ Kaufman KR, Bagayogo IP, Kaufman IH, Das A (2014). "Zolpidem and anoxic encephalopathy: prolonged treatment response.". Ann Clin Psychiatry. 26 (1): 70–2. PMID 24501733.
- ↑ Autret K, Arnould A, Mathieu S, Azouvi P (2013). "Transient improvement of poststroke apathy with zolpidem: a single-case, placebo-controlled double-blind study.". BMJ Case Rep. 2013: bcr2012007816. doi:10.1136/bcr-2012-007816. PMC 3604023
 . PMID 23396925.
. PMID 23396925.
Further reading
- Joel Lamoure RPh. BScPhm.,FASCP. "How Is Zolpidem Dependence Managed?". Medscape Pharmacists Ask the Expert. WebMD. Retrieved 2010-03-05. (registration required (help)).
- "Prescription Sleep Aid AMBIEN CR". Sanofi-Aventis. Ambien CR official website. April 2013. Retrieved 2009-05-21.
- "Ambien Cr (zolpidem tartrate) Tablet, Coated". DailyMed. U.S. National Library of Medicine, National Institutes of Health, Health & Human Services. Archived from the original on June 9, 2009. Retrieved 2009-05-21.
- U.S. National Library of Medicine: Drug Information Portal – Zolpidem
External links
![]() Media related to Zolpidem at Wikimedia Commons
Media related to Zolpidem at Wikimedia Commons