Dibenzepin
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | N06AA08 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25% (Oral) |
| Protein binding | 80% |
| Metabolism | Hepatic |
| Biological half-life | 5 hours |
| Excretion | Urine (80%), Feces (20%) |
| Identifiers | |
| |
| CAS Number |
4498-32-2 |
| PubChem (CID) | 9419 |
| ChemSpider |
9048 |
| UNII |
510SJZ1Y6L |
| KEGG |
D07812 |
| ChEMBL |
CHEMBL1442422 |
| Chemical and physical data | |
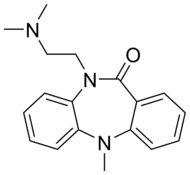
| Formula | C18H21N3O |
| Molar mass | 295.379 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Dibenzepin (Noveril, Anslopax, Deprex, Ecatril, Neodit, Victoril) is a tricyclic antidepressant (TCA) used widely throughout Europe for the treatment of depression.[1][2][3] It has similar efficacy and effects relative to other TCAs like imipramine but with fewer side effects.[4][5][6][7] Dibenzepin acts as a norepinephrine reuptake inhibitor, potent antihistamine, and weak anticholinergic.[4][5][8] It lacks any 5-HT2 antagonistic properties.[9]
Chronic pain
Like other tricyclic antidepressants, dibenzepin may have potential use in the treatment of chronic neuropathic pain.
Overdose
As tricyclic antidepressants have a relatively narrow therapeutic index, the likelihood of overdose (both accidental and intentional) is fairly high and should be considered carefully by the prescribing physician prior to patient use. Symptoms of overdose are similar to those of other tricyclic antidepressants, with cardiac toxicity (due to inhibition of sodium and calcium channels) generally occurring before the threshold for serotonin syndrome is reached. Due to this risk, tricyclic antidepressants are rarely selected as the first line treatment for depression.
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ↑ Sittig, Marshall (1988). Pharmaceutical manufacturing encyclopedia. Park Ridge, N.J., U.S.A: Noyes Publications. ISBN 0-8155-1144-2.
- ↑ Beresewicz M, Bidzińska E, Koszewska I, Puzyński S (1991). "[Results of using tricyclic antidepressive drugs in the treatment of endogenous depression (comparative analysis of 7 drugs)]". Psychiatria Polska (in Polish). 25 (3-4): 13–8. PMID 1687987.
- 1 2 "Novartis (dibenzepin) - Prescribing Information" (PDF).
- 1 2 Paloucek, Frank P.; Leikin, Jerrold B. (2007). Poisoning and Toxicology Handbook, Fourth Edition (Poisoning and Toxicology Handbook (Leiken & Paloucek's)). Informa Healthcare. ISBN 1-4200-4479-6.
- ↑ Gowardman M, Brown RA (March 1976). "Dibenzepin and amitriptyline in depressive states: comparative double-blind trial". The New Zealand Medical Journal. 83 (560): 194–7. PMID 6928.
- ↑ Baron DP, Unger HR, Williams HE, Knight RG (April 1976). "A double blind study of the antidepressants dibenzepin (Noveril) and amitriptyline". The New Zealand Medical Journal. 83 (562): 273–4. PMID 8749.
- ↑ Rehavi M, Maayani S, Goldstein L, Assael M, Sokolovsky M (August 1977). "Antimuscarinic properties of antidepressants: dibenzepin (Noveril)". Psychopharmacology. 54 (1): 35–8. doi:10.1007/BF00426538. PMID 20647.
- ↑ Closse A, Jaton AL (July 1984). "Investigation of the influence of lithium upon the down-regulation of serotonin2 receptors in rat frontal cortex induced by long-term treatment with dibenzepin, an antidepressant without appreciable affinity to serotonin2 receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 326 (4): 291–3. doi:10.1007/bf00501432. PMID 6148707.