Vesicular monoamine transporter 1
| View/Edit Human | View/Edit Mouse |
Vesicular monoamine transporter 1 (VMAT1) also known as chromaffin granule amine transporter (CGAT) or solute carrier family 18 member 1 (SLC18A1) is a protein that in humans is encoded by the SLC18A1 gene. VMAT1 is an integral membrane protein, which is embedded in synaptic vesicles and serves to transfer monoamines, such as norepinephrine, epinephrine, dopamine, and serotonin, between the cytosol and synaptic vesicles.[4] SLC18A1 is an isoform of the vesicular monoamine transporter.
Discovery
The idea that there must be specific transport proteins associated with the uptake of monoamines and acetylcholine into vesicles developed due to the discovery of specific inhibitors which interfered with monoamine neurotransmission and also depleted monoamines in neuroendocrine tissues.[4] VMAT1 and VMAT2 were first identified in rats upon cloning CDNAs for proteins which gave non-amine accumulating recipient cells the ability to sequester monoamines.[5] Subsequently, human VMATs were cloned using human cDNA libraries with the rat homologs as probes, and heterologous-cell amine uptake assays were performed to verify transport properties.[6]
Structure
Across mammalian species, VMATs have been found to be structurally well conserved; VMAT1s have an overall sequence identity exceeding 80%. However, there exists only a 60% sequence identity between the human VMAT1 and VMAT2.[7]
VMAT1 is an acidic glycoprotein with an apparent weight of 40 kDa.[8] Although the crystallographic structure has not yet been fully resolved, VMAT1 is known to have either twelve transmembrane domains (TMDs), based on Kyte-Doolittle hydrophobicity scale analysis[6] or ten TMDs, based on MAXHOM alignment. MAXHOM alignment was determined using the "profile-fed neural network systems from Heidelberg" (PHD) program.[4] The main difference between these two models arises from the placement of TMDs II and IV in the vesicle lumen or the cytoplasm.
Localization
Cell types
VMATs are found in a variety of cell types throughout the body, however, VMAT1 is found exclusively in neuroendocrine cells, in contrast to VMAT2, which is also found in the PNS and CNS. Specifically, VMAT1 is found in chromaffin cells, enterochromaffin cells, and small intensely fluorescent cells (SIFs).[9] Chromaffin cells are responsible for releasing the catecholamines (norepinephrine and epinephrine) into systemic circulation. Enterochromaffin cells are responsible for storing serotonin in the gastrointestinal tract. SIFs are interneurons associated with the sympathetic nervous system which are managed by dopamine.
Vesicles
VMAT1 is found in both large dense-core vesicles (LDCVs) as well as in small synaptic vesicles (SSVs). This was discovered via studying rat adrenal medulla cells (PC12 cells).[10] LDCVs are 70-200 nm in size and exist throughout the neuron (soma, dendrites, etc.). SSVs are much smaller (usually about 40 nm) and typically exist as clusters in the presynaptic cleft.
Function
Active transport of monoamines
Driving force
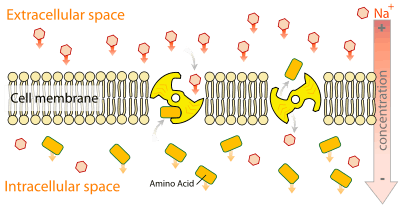
The active transport of monoamines from the cytosol into storage vesicles operates against a large (>105) concentration gradient. Secondary active transport is the type of active transport used, meaning that VMAT1 is an antiporter. This transport is facilitated via proton gradient generated by the protein proton ATPase. The inward transport of the monoamine is coupled with the efflux of two protons per monoamine.[11] The first proton is thought to cause a change in VMAT1's conformation, which pushes a high affinity amine binding site, to which the monoamine attaches. The second proton then causes a second change in the conformation which pulls the monoamine into the vesicle and greatly reduces the affinity of the binding site for amines. A series of tests suggest that His419, located between TMDs X and XI, plays the key role in the first of these conformational changes, and that Asp431, located on TMD XI, does likewise during the second change.[8]
Inhibition
Several reuptake inhibitors of VMATs are known to exist, including reserpine (RES), tetrabenazine (TBZ), dihydrotetrabenazine (DTBZOH), and ketanserin (KET). It is thought that RES exhibits competitive inhibition, binding to the same site as the monoamine substrate, as studies have shown that it can be displaced via introduction of norepinephrine. TBZ, DTBZOH, and KET are thought to exhibit non-competitive inhibition, instead binding to allosteric sites and decreasing the activity of the VMAT rather than simply blocking its substrate binding site.[8] It has been found that these inhibitors are less effective at inhibiting VMAT1 than VMAT2, and the inhibitory effects of the tetrabenazines on VMAT1 is negligible.[9]
Clinical significance
Pancreatic cancer
The expression of VMAT1 in healthy endocrine cells was compared to VMAT1 expression in infants with hyperinsulinemic hypoglycemia and adults with pancreatic endocrine tumors.[12] Through immunohistochemistry (IHC) and in situ hybridization (ISH), they found VMAT1 and VMAT2 were located in mutually exclusive cell types, and that in insulinomas VMAT2 activity disappeared, suggesting that if only VMAT1 activity is present in the endocrine system, this type of cancer is likely.
Digestive system
VMAT1 also has effects on the modulation of gastrin processing in G cells. These intestinal endocrine cells process amine precursors, and VMAT1 pulls them into vesicles for storage. The activity of VMAT1 in these cells has a seemingly inhibitory effect on the processing of gastrin. Essentially, this means that certain compounds in the gut can be taken into these G cells and either amplify or inhibit the function of VMAT1, which will impact gastrin processing (conversion from G34 to G17).[13]
Additionally, VMAT1 is known to play a role in the uptake and secretion of serotonin in the gut. Enterochromaffin cells in the intestines will secrete serotonin in response to the activation of certain mechanosensors.[14] The regulation of serotonin in the gut is critically important, as it modulates appetite and controls intestinal contraction.
Protection against hypothermia
Presence of VMAT1 in cells has been shown to protect them from the damaging effects of cooling and rewarming associated with hypothermia.[15] Experiments were carried out on aortic and kidney cells and tissues. Evidence was found that an accumulation of serotonin using VMAT1 and TPH1 allowed for the subsequent release of serotonin when exposed to cold temperatures. This allows cystathionine beta synthase (CBS) mediated generation of H2S. The protection against the damage caused by hypothermia is due to a reduction in the generation of reactive oxygen species (ROS), which can induce apoptosis, due to the presence of H2S.[16]
Mental disorders
VMAT1 (SLC18A1) maps to a shared bipolar disorder(BPD)/schizophrenia locus, which is located on chromosome 8p21.[17] It is thought that disruption in transport of monoamine neurotransmitters due to variation in the VMAT1 gene may be relevant to the etiology of these mental disorders. One study looked at a population of European descent, examining the genotypes of a bipolar group and a control group. The study confirmed expression of VMAT1 in the brain at a protein and mRNA level, and found a significant difference between the two groups, suggesting that, at least for people of European descent, variation in the VMAT1 gene may confer susceptibility.[17] A second study examined a population of Japanese individuals, one group healthy and the other schizophrenic. This study resulted in mostly inconclusive findings, but some indications that variation in the VMAT1 gene would confer susceptibility to schizophrenia in Japanese women.[18] While these studies provide some promising insight into the cause of some of the most prevalent mental disorders, it is clear that additional research will be necessary in order to gain a full understanding.
References
- ↑ "Drugs that physically interact with Chromaffin granule amine transporter view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 Eiden LE, Schäfer MK, Weihe E, Schütz B (February 2004). "The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflugers Arch. 447 (5): 636–40. doi:10.1007/s00424-003-1100-5. PMID 12827358.
- ↑ Erickson JD, Eiden LE, Hoffman BJ (November 1992). "Expression cloning of a reserpine-sensitive vesicular monoamine transporter". Proc. Natl. Acad. Sci. U.S.A. 89 (22): 10993–7. doi:10.1073/pnas.89.22.10993. PMC 50469
 . PMID 1438304.
. PMID 1438304. - 1 2 Erickson JD, Eiden LE (December 1993). "Functional identification and molecular cloning of a human brain vesicle monoamine transporter". J. Neurochem. 61 (6): 2314–7. doi:10.1111/j.1471-4159.1993.tb07476.x. PMID 8245983.
- ↑ Eiden LE, Schäfer MK, Weihe E, Schütz B (February 2004). "The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflugers Arch. 447 (5): 636–40. doi:10.1007/s00424-003-1100-5. PMID 12827358.
- 1 2 3 Wimalasena K (July 2011). "Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry". Med Res Rev. 31 (4): 483–519. doi:10.1002/med.20187. PMC 3019297
 . PMID 20135628.
. PMID 20135628. - 1 2 Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E (May 1996). "Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter". Proc. Natl. Acad. Sci. U.S.A. 93 (10): 5166–71. doi:10.1073/pnas.93.10.5166. PMC 39426
 . PMID 8643547.
. PMID 8643547. - ↑ Liu Y, Schweitzer ES, Nirenberg MJ, Pickel VM, Evans CJ, Edwards RH (December 1994). "Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells". J. Cell Biol. 127 (5): 1419–33. doi:10.1083/jcb.127.5.1419. PMC 2120259
 . PMID 7962100.
. PMID 7962100. - ↑ Parsons SM (December 2000). "Transport mechanisms in acetylcholine and monoamine storage". The FASEB Journal. 14 (15): 2423–2434. doi:10.1096/fj.00-0203rev. PMID 11099460.
- ↑ Anlauf M, Eissele R, Schäfer MK, Eiden LE, Arnold R, Pauser U, Klöppel G, Weihe E (August 2003). "Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors" (PDF). J. Histochem. Cytochem. 51 (8): 1027–40. doi:10.1177/002215540305100806. PMID 12871984.
- ↑ Hussain I, Bate GW, Henry J, Djali P, Dimaline R, Dockray GJ, Varro A (June 1999). "Modulation of gastrin processing by vesicular monoamine transporter type 1 (VMAT1) in rat gastrin cells". J. Physiol. (Lond.). 517 (2): 495–505. doi:10.1111/j.1469-7793.1999.00495.x. PMC 2269351
 . PMID 10332097.
. PMID 10332097. - ↑ Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM, Kidd M (February 2012). "The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion". Am. J. Physiol. Gastrointest. Liver Physiol. 302 (3): G397–405. doi:10.1152/ajpgi.00087.2011. PMC 3287403
 . PMID 22038827.
. PMID 22038827. - ↑ Talaei F, Schmidt M, Henning RH (2012). "Induction of VMAT-1 and TPH-1 expression induces vesicular accumulation of serotonin and protects cells and tissue from cooling/rewarming injury". PLoS ONE. 7 (1): e30400. doi:10.1371/journal.pone.0030400. PMC 3257274
 . PMID 22253933.
. PMID 22253933. - ↑ Talaei F, Bouma HR, Van der Graaf AC, Strijkstra AM, Schmidt M, Henning RH (2011). "Serotonin and dopamine protect from hypothermia/rewarming damage through the CBS/H2S pathway". PLoS ONE. 6 (7): e22568. doi:10.1371/journal.pone.0022568. PMC 3144905
 . PMID 21829469.
. PMID 21829469. - 1 2 Lohoff F, Dahl J, Ferraro T, Arnold S, Gallinat J, Sander T, Berrettini W (December 2006). "Variations in the vesicular monoamine transporter type 1 gene (VMAT1/SLC18A1) are associated with bipolar I disorder". Neuropsychopharmacology. 31 (12): 2739–2747. doi:10.1038/sj.npp.1301196. PMC 2507868
 . PMID 16936705.
. PMID 16936705. - ↑ Richards M, Iijima Y, Kondo H, Shizuno T, Hori H, Arima K, Saitoh O, Kunugi H (2006). "Association study of the vesicular monoamine transporter 1 (VMAT1) gene with schizophrenia in a Japanese population". Behav Brain Funct. 2: 39. doi:10.1186/1744-9081-2-39. PMC 1697819
 . PMID 17134514.
. PMID 17134514.
External links
- Vesicular Monoamine Transporter 1 at the US National Library of Medicine Medical Subject Headings (MeSH)